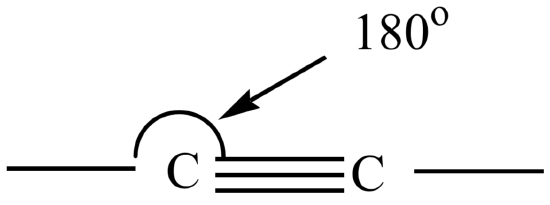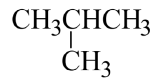18: Introduction to the Structures and Isomerism of Simple Organic Molecules- Description and Modeling (Experiment)
- Page ID
- 95998
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Part I: To learn the structures of and construct models for simple organic molecules, and to draw their projection and perspective formulae.
- Part II: To classify, recognize and construct models of different types of isomers of organic compounds.
Almost all compounds that contain carbon are known as organic compounds. Most organic compounds also contain hydrogen. Organic compounds that contain only carbon and hydrogen atoms are classified as hydrocarbons. Structures and simple physical properties of hydrocarbons will be discussed in Part I. Some organic compounds also contain other nonmetals (referred to as heteroatoms) such as oxygen, nitrogen, the halogens, sulfur, and phosphorus. These compounds are known as hydrocarbon derivatives.
Based on their structures and properties, organic compounds are also classified according to groups of atoms within the molecule called functional groups. Each functional group is characterized by the type of bonds and/or heteroatoms it contains. The most common functional groups are the alkanes, alkenes, alkynes, aromatic hydrocarbons, organic halides, alcohols, aldehydes, ketones, esters, carboxylic acids, amines and amides. The first four of these functional groups contain only carbon and hydrogen atoms and therefore belong to the class of hydrocarbons.
Molecules that have the same molecular formula but are not identical are called conformers or isomers. Conformers differ only by the angle of rotation about a single bond(s) however isomers have different structural or spatial arrangements.
In part II different classes of conformers and isomers will be discussed and molecular models will be used to visualize their three-dimensional structures.
Part I: Hydrocarbons
The four classes of hydrocarbons are alkanes, alkenes, alkynes and aromatic hydrocarbons. Alkanes and alkenes and alkynes are further classified as aliphatic hydrocarbons.
Names of hydrocarbons use a root name that indicates the number of carbon atoms. Then appropriate suffixes and/or prefixes are added to complete the name. For example: heptane indicate a seven (hepta-) alkane (-ane). The number of carbon atoms in a chain or ring is indicated as follows:
| Number of C atoms | Root Name | Number of C atoms | Root Name |
|---|---|---|---|
| 1 | meth- | 7 | hept- |
| 2 | eth- | 8 | oct- |
| 3 | prop- | 9 | non- |
| 4 | but- | 10 | dec- |
| 5 | pent- | 11 | undec- |
| 6 | hex- | 12 | dodec- |
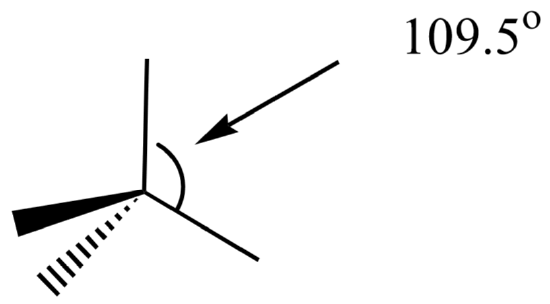
Alkanes contain single bonds only. Each carbon in an alkane has a tetrahedral geometry with a bond angle of about 109.5o. Carbon atoms in alkanes are sp3 hybridized.
The relationship between the number of carbons and hydrogens in alkanes is summarized by the general formula \(\text{C}_{n}\text{H}_{2n+2}\), where \(n\) = # of carbons.
The structures and names of the straight chain alkanes containing one to six carbons are as follows:
Notice that their names end in –ane.
- \(\ce{CH4}\) methane
- \(\ce{CH3CH3}\) ethane
- \(\ce{CH3CH2CH3}\) propane
- \(\ce{CH3CH2CH2CH3}\) butane
- \(\ce{CH3CH2CH2CH2CH3}\) pentane
- \(\ce{CH3CH2CH2CH2CH2CH3}\) hexane. And so on.
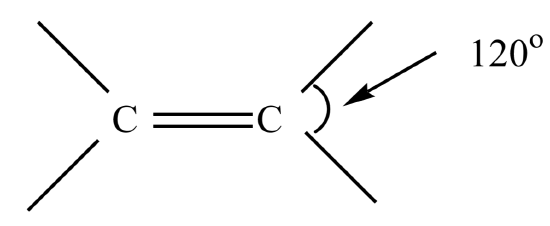
Alkenes contain one or more double bonds. The geometry around the double bonded carbons is trigonal planar with a bond angle of 120o around the carbon. The double bonded carbons in alkenes are sp2 hybrized.
The relationship between the number of carbons and hydrogens in alkenes is summarized by the general formula \(\text{C}_{n}\text{H}_{2n}\), \(n\) = # of carbon atoms.
The structures and names of the simplest straight chain alkenes (with one double bond between the first and the second carbons) containing two to six carbons are as follows:
Notice that their names end in –ene.
- \(\ce{CH2}\)=\(\ce{CH2}\) ethene
- \(\ce{CH2}\)=\(\ce{CHCH3}\) 1-propene
- \(\ce{CH2}\)=\(\ce{CHCH2CH3}\) 1-butene
- \(\ce{CH2}\)=\(\ce{CHCH2CH2CH3}\) 1-pentene
- \(\ce{CH2}\)=\(\ce{CHCH2CH2CH2CH3}\) 1-hexene. And so on.
Alkynes contain one or more triple bonds. The geometry around the triple bonded carbons is linear with a bond angle of 180o. The triple bonded carbons in alkynes are sp hybridized.
The relationship between the number of carbons and hydrogens in alkynes is summarized by the general formula \(\text{C}_{n}\text{H}_{2n-2}\), \(n\) = # of carbon atoms.
The structures and names of the simplest straight chain alkynes (with one triple bond between the first and the second carbons) are as follows:
Notice that their names end in –yne.
- \(\ce{CH}\)≡\(\ce{CH}\) ethyne
- \(\ce{CH}\)≡\(\ce{CCH3}\) propyne
- \(\ce{CH}\)≡\(\ce{CCH2CH3}\) 1-butyne
- \(\ce{CH}\)≡\(\ce{CCH2CH2CH3}\) 1-pentyne
- \(\ce{CH}\)≡\(\ce{CCH2CH2CH2CH3}\) 1-hexyne And so on.
Branched and cyclic hydrocarbons: Carbon atoms in organic compounds not only are arranged in a straight chain (each carbon is bonded to maximum of two other carbons) but can also be arranged in branched chain (each carbon can be bonded to three or four other carbons) or a cyclic chain (the carbons are arranged to form a ring).
Branched aliphatic hydrocarbons can be named using common (old) names (specially the small molecular weights) or are named systematically by following a set of rules for naming.
Cyclic aliphatic hydrocarbons are simply named by adding a prefix cyclo- in front of the name of their analogous open chain hydrocarbon.
For example an alkane with 4 carbons can be arranged in:
Straight chain:
\[\ce{CH3CH2CH2CH3}\]
- butane
Branched chain:
- isobutane (common name)
Cyclic chain:
- cyclobutane
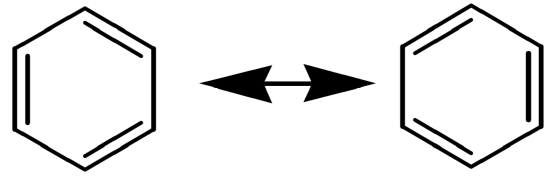
Aromatic hydrocarbons refer to structures where carbons are bonded to each other forming a planar cyclic carbon chain (ring) and contain delocalized \(\pi\) (pi) bonds, as suggested by the resonance structures below. The most common aromatic compounds are benzene (\(\ce{C6H6}\)) and its derivatives (a benzene ring with substituent(s) attached to it). The carbons in aromatic hydrocarbons are sp2 hybridized and the C-C-C and C-C-H bond angles are 120o.
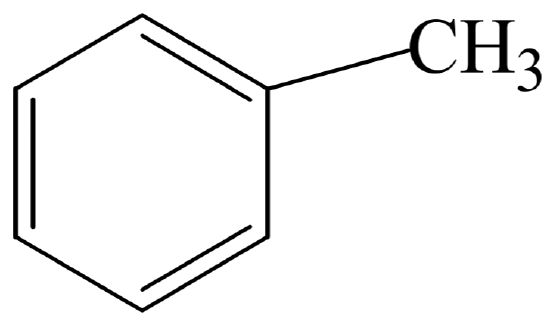
Benzenes derivates are named by using their common names or named by using the name of the subsitituent + benzene (eg methylbenzene)
Benzene
Also represented as:
A benzene derivative:
Methylbenzene (toluene)
Saturated and unsaturated hydrocarbons: Carbon can form a maximum of four single bonds in a compound. Since each carbon in an alkane contains four single bonds with maximum number of hydrogens possible, alkanes are called saturated hydrocarbons. However alkenes, alkynes and aromatic hydrocarbons contain \(\pi\)-bonded (double or triple bonds) carbons and contain less than the maximum number of hydrogens. Therefore they are called unsaturated hydrocarbons. The pi-bonds in unsaturated organic compounds can be converted to single bonds by adding additional atoms to form saturated organic compounds.
Intermolecular force between hydrocarbons and physical properties: The primary intermolecular forces between hydrocarbons are London dispersion forces. London forces are relatively weak for small molecules but increase in magnitude with increasing molecular size. Therefore at room temperature hydrocarbons containing four or less carbons exist as gases; hydrocarbons containing five to twelve carbons exist as liquids; hydrocarbons containing more than twelve carbons generally exist as solids.
Part II: Conformers and Isomers
Different orientations of the same molecule that differ only by the angle of rotation about a single bond or bonds are called conformers. Conformers look different, but are really the same compound since one can be converted to another simply by rotation about a single bond.
Different compounds with the same molecular formula that have different structural or spatial arrangements are called isomers.
Conformers (also called conformations or conformational isomers)
The different arrangements of atoms can arise by simple rotation around single bonds. These different arrangements are said to be conformers. For most molecules at room temperature there is sufficient thermal energy to convert one conformer into another by rotation around a single bond. For this reason it said that there is “free rotation” around a single bond. Sometimes sets of conformers for a particular molecule are given special names describing their orientations. The compound will exist as equilibrium mixture of conformers.
The anti and eclipsed conformers of ethane:
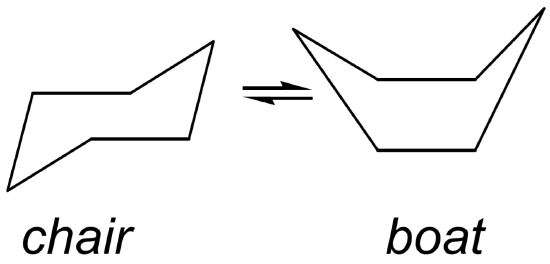
The chair and the boat conformers of cyclohexane:
Isomers
Different arrangements of atoms in a set of molecules that cannot be interconverted by simple rotation around a single bond are called isomers. Interconversion of isomers is much more restricted and takes much more energy than interconversion of conformers. Enough energy to break one or more \(\pi\) or \(\sigma\) bonds is needed for interconversion of isomers. Therefore, they are different compounds and exhibit different properties.
There are two main classes of isomers called constitutional (structural) isomers and stereoisomers. These types of isomers not only in organic compounds but also in inorganic compounds (coordination complexes) discussed in the second semester of general chemistry.
Constitutional (Structural) Isomers
Isomers with different structural arrangements are called constitutional isomers. Constitutional isomers can differ from each other by their sequence of atoms, their sequence of bonds or their types of bonds. The following illustrate different types of constitutional isomers.
Different sequence of atoms (same functional group).
\(\ce{C4H10}\)
\(\ce{CH3CH2CH2CH3}\)
butane isobutene (methylpropane)
- • different sequence of bonds (different location of functional group)
CH3CHCH3
o C4H8
CH2 CHCH 2CH3 CH3CH 1-butene 2-butene
• different types of bonds (different functional group) o C2H6O
CH3OCH3 CH3CH2OH
CHCH3
Stereoisomers
Isomers that differ from each other by the way their atoms are arranged in space are called stereoisomers. There are two classes of stereoisomers: enantiomers and diastereomers
•Enantiomers (optical isomers)
An object or a compound that does not contain a plane or a center of symmetry is said to be chiral. Chiral objects have the property that they are not superimposable (exactly overlap) on their mirror images. A common example of a pair of enantiomers are your hands. They are mirror images of each other, but are not superimposable. Thus, chiral compounds or object are said to exhibit “handedness”.
Just as certain objects are chiral, certain molecules and ions are also chiral. The most common chiral organic compounds contain at least one tetrahedral carbon that is bonded to four different groups. A tetrahedral carbon that contains four different groups is said to have a chiral or stereogenic center. In most cases, compounds containing chiral carbons are not superimposable on their mirror images. If the molecule or ion is chiral and therefore is not superimposable on its mirror image, then the molecule and its mirror image are said to be enantiomers (or optical isomers) of each other.
Enantiomers have identical physical and chemical properties except for their effect on plane polarized light and their reactions with other chiral (asymmetric) compounds. A pair of enatiomers rotate plane polarized light in opposite directions. For this reason, enantiomers are also known as optical isomers. Biological molecules generally exhibit optical isomerism and this property can be very important in biological reactions. For example, different optical isomers can have very different tastes, odors and toxicities.
Objects or molecules that are identical to their mirror images and are therefore superimposable on their mirror images are said to be achiral (not chiral). Examples include CH2Cl2, or any tetrahedral carbon with at least two identical groups bonded to it.
• Diastereomers
There are two types of diatereomers:
i) Geometric isomers (also called cis/trans isomers)
ii) stereomisomers that have two or more chiral centers but are not mirror images (optional)
i) Geometric Isomers
Geometric isomers in organic compounds have different orientations of atoms or groups across a double bond or a ring. If the same types of atoms or groups of atoms are attached to the same side of the double bond or a ring then the arrangement is referred as cis- isomer.
If the same types of substituents are attached to the opposite side of the double bond or a ring then the arrangement is referred to as trans- isomer.
• Trans-2-butene CH3
H
CC
H CH3
Geometric isomerism arises because there is no “free” rotation around a double bond or within the carbons in a ring. Bonds have to be broken to convert one isomer into the other. ii) stereomisomers that have two or more chiral centers but are not enatiomers (optional)
Different spatial arrangements around the chiral carbons can lead to stereoisomers that are not mirror images. Compounds that contain 2 or more chiral carbon atoms but that are not mirror images of each other are said to be diastereomers. Molecules that contain two or more chiral centers can therefore have both enantiomers and diastereomers. This type of isomerism is common in carbohydrate chemistry.
A projection diagram called a Fischer projection best represents molecules that have two or more chiral centers and their isomers. Fischer projections show three-dimensional arrangement of the bonds in a molecule. In Fisher projections bonds coming out of the plane are drawn with horizontal lines and bonds that are going into the plane are drawn with vertical lines. The tetrahedral carbons are represented by the intersection of the horizontal and vertical lines.
Fischer projections of D-glucose and D-galactose are shown below.
•
H HO H H
A & B and B & C are diastereomers of each other. However A and C are enantiomers of each other.
CHO
CH2OH CH2OH CH2OH ABC
CHO CHO
H OHHO H
HO HH OH
OHHO HHO H
OHH OHHO H
OH
H
D-glucose D-galactose L- glucose
Unlike pairs of enantiomers, diastereomers have different physical properties beyond their effects on plane polarized light.
Introduction to the Structures and Isomerism of Page 8 of 10 Simple Organic Molecules: Description and Modeling
Chemistry 11
Santa Monica College
Summary of Conformers and Isomers
Different arrangements of atoms in a molecule or molecules
conformers
(different arrangements caused by single bond rotation
isomers
(different arrangement caused by breaking of bonds)
constitutional (structural) isomers stereoisomers
(different connectivity) (same connectivity, different spatial arrangement)
enantiomers
(mirror images, not superimposable)
diastereomers
containing chiral centers
Molecular models will be used to illustrate the three-dimensional structures of simple hydrocarbons and different types of isomers. The model kits contain different colored balls and different size sticks. Three-dimensional models will be constructed from these balls and sticks.
geometric isomers
Using Molecular Model Kits
Different colored balls represent different atoms. Commonly the black balls represent carbon atoms, white balls represent hydrogen atoms and red balls represent oxygen atoms. The halogens (chlorine, iodine, and bromine) are usually represented by the green balls. Blue balls represent nitrogen atoms. However it is also possible to determine which balls to use to represent an atom by just looking at the number of holes in the balls. The holes represent the number of bonds these atoms generally form. For example carbon forms four bonds and therefore to use a ball that has four holes is used, oxygen forms two bonds and therefore the red one that has two holes is used. Hydrogen forms one bond and therefore the white ball with the one hole is used. The halogens usually form one bond so the green balls with one hole are used. The blue ball that is used for nitrogen is an exception since it contains four holes and nitrogen usually forms three bonds. Therefore remaining hole can represent the lone pair on the atom.
The plastic sticks in the kits represent bonds. There are two different sized sticks. The short, stiff sticks are used to represent single bonds and the long flexible sticks are used for double and triple bonds. In order to make one double bond two flexible sticks are needed to connect the two adjacent atoms and to make a triple bond three flexible sticks are used to connect the two adjacent atoms.
Introduction to the Structures and Isomerism of Page 9 of 10 Simple Organic Molecules: Description and Modeling
Chemistry 11 Santa Monica College
Molecular models will be constructed for each molecule. Projection structures as well as three dimensional perspective diagrams will be drawn. The projection structure is just the Lewis structure in which each pair boding electrons is represented as a line. Three dimensional perspective drawings require a different drawing technique to identify bond orientation. A solid straight lines ( ) represent bonds lying in the plane of the paper, a wedge-shaped line
constructed of hatch marks ( ) represents bonds that project down into the paper, and solid wedge-shaped line ( ) represents bonds that project up out of the paper, toward the
viewer.
• Projection and perspective diagram of CH4
H HCH
H H
HH
H
projection perspective
It is important to be able to visualize these three-dimensional structures in your mind without the aid of molecular models. If you need further practice with these models you can purchase them from the SMC bookstore or you can you be creative and make home made models using materials that are available in your home.
Procedure
Materials: molecular model kit. Obtain one kit per student from your instructor or the stockroom. For each compound listed in the data sheet:
- Construct a model.
- Draw projection and perspective pictures in the space provided on the data sheet.
- Answer the questions for each.
- Show your instructor the models to verify their correctness
Prelab Assignment for Introduction to the Structures and Isomerism of Simple Organic Molecules: Description and Modeling
Be sure to read the Background section before answering these questions.
- Draw the structures and give the names of the straight chain alkanes containing seven to twelve carbons.
- Draw the structures and give the names of the simplest straight chain alkenes (the double bond between C1 – C2) containing seven to twelve carbons.
- Draw the structures and give the names of the simplest straight chain (the triple bond between C1 – C2) alkynes containing seven to twelve carbons.
- Draw all three possible structures of benzene containing two methyl (-CH3) substituents. Give two additional examples of constitutional isomers.
- Draw a 3-D structure of a molecule with a molecular formula of C4H9Cl containing one chiral center and draw its mirror image.
- Draw a pair of cyclic cis-trans isomers for compounds with the molecular formula C4H6Br2.
- Introduction to the Structures and Isomerism of Page 1 of 1 Simple Organic Molecules: Description and Modeling
- Lab Report for Introduction to the Structures and Isomerism of Simple Organic Molecules: Description and Modeling
Part I: Hydrocarbons Alkanes
1. CH4 (methane)
|
Projection Drawing: |
Perspective Drawing: |
2. CH3CH3 (ethane)
|
Projection Drawing: |
Perspective Drawing: |
Alkenes
3. CH2CH2 (ethene or ethylene)
|
Projection Drawing: |
Perspective Drawing: |
4. CH2CHCH3 (propene or propylene)
|
Projection Drawing: |
Perspective Drawing: |
Alkynes
5. CHCH (ethyne or acetylene)
|
Projection Drawing: |
Perspective Drawing: |
6. CHCCH3 (propyne or methylacetylene)
|
Projection Drawing: |
Perspective Drawing: |
Aromatic hydrocarbons
7. C6H6 (benzene)
|
Projection Drawing: |
Perspective Drawing: |
8. o-C6H4Cl2 (ortho-dichlorobenzene or 1,2-dichlorobenzene).
|
Projection Drawing: |
Perspective Drawing: |
Part II: Conformers and Isomers
9. Eclipsed and Anti C2H6.
Perspective drawings of both conformers:
10. Chair and Boat Conformers of Cyclohexane (C6H12). Note it is impossible to place all the carbons in the same plane without straining the bonds. Take two opposite carbons and pull both of them up to make one conformation and then pull one of them down to make the other conformation.
Perspective Drawings:
11. Reconstruct the ethane molecules from part A. Replace one of the H atoms in the ethane model with a Br atom, to form ethyl bromide CH3CH2Br.
|
Projection Drawing: |
Perspective Drawing: |
12. Replace one H atom in CH3CH2Br by another Br atom to form a model of a compound with the formula C2H4Br2.
|
Projection drawings of all possible isomers: |
Perspective drawings of all possible isomers: |
13. An alkane with a formula C3H8 (propane).
|
Projection drawings of all possible isomers: |
Perspective drawings of all possible isomers: |
a. How many possible isomers exist for C3H8?
14. The alkanes C4H10 and C5H12.
Projection drawings of all isomers of C4H10 :
Projection drawings of all isomers of C5H12 :
Introduction to the Structures and Isomerism of Simple Organic Molecules: Description and Modeling
Page 7 of 10
Chemistry 11 Santa Monica College
15. Construct models of o-C6H4Cl2 (1,2-dichlorobenzene), m-C6H4Cl2 (1,3-dichlorobenzene) and p-C6H4Cl2, p-C6H4Cl2 (1,4-dichlorobenzene)
Projection drawings of all isomers of C5H12 :
Projection drawings of all isomers of C6H4Cl2 (dichlorobenzene):
16. Enantiomers of CHBrIF
Perspecitve drawings of both enantiomers:
a. Make models of both enantiomers (make one model then make its mirror image.)
Introduction to the Structures and Isomerism of Page 8 of 10 Simple Organic Molecules: Description and Modeling
Chemistry 11
Santa Monica College
b.
c.
Can the two models be superimposed?
What type of isomerism do these models represent?
17. Geometric Isomers of ClCH = CHCl (1,2-dichloroethene)
Perspective drawings of both isomers:
Introduction to the Structures and Isomerism of Page 9 of 10 Simple Organic Molecules: Description and Modeling
Chemistry 11 Santa Monica College
18. Diastereomers of CH3CH(OH)CH(OH)CH2CH3 (optional)
Fischer projection of one isomer:
Fischer projection of mirror image:
c. Are these molecules superimposable?
Fischer projection of third isomer:
f. What is the relationship of the third isomer to the first two isomers you made?
Introduction to the Structures and Isomerism of Page 10 of 10 Simple Organic Molecules: Description and Modeling
- Predict the angle of HCH bond.
- Name of geometry.
- Name the hybridization of the carbon.
- Predict the angels of HCH and HCC bonds.
- Name of the geometry around each carbon.
- Name the hybridization of the carbon.
- Predict the angle HCH and HCC.
- Name of the geometry around each carbon.
- Name the hybridization of each carbon atom.
- Predict the angle HCH (the first carbon).
- Predict the angle HCC (the first and the second carbon).
- Predict the angle HCH (the third carbon).
- Predict the angle HCC.
- Name the molecular geometry about each carbon.
- Name the hybridization of the carbon atoms.
- Predict the angel HCC (the first carbon).
- Predict the molecular geometry around each carbon.
- Predict the angle HCC.
- Predict the angle CCC.
- Name the geometry around each carbon atom.
- Name the hybridization of each carbon.
- Predict the angle CCC.
- Predict the angle ClCC.
- Indicate the position of the two chlorine atoms on the benzene ring.
- What is the relationship between the above two structures?
- How many different eclipsed or anti conformers can you make by rotating around the C – C bond?
- Can you interconvert one conformer into the other without breaking any bonds?
- Explain why these represent conformers and not isomers.
- Does replacing different hydrogen atoms in CH3CH3 to produce CH3CH2Br give you different isomers? (i.e. Are all the hydrogen atoms equivalent?)
- How many isomers of CH3CH2Br can you construct?
- Make a model of one other conformer of CH3CH2Br.
- Did you need to break any bonds to form the second conformer from the first?
- Does replacing different hydrogen atoms in CH3CH2Br to produce C2H4Br2 give different isomers? (i.e. Are all the hydrogen atoms equivalent?)
- If there are multiple isomers, what type of isomerism do they represent?
- How many possible isomers exist for each?
- What type of isomerism do they represent?
- Can you convert one into another without breaking any bonds?
- What type isomerism do these represent?
- Make two models that have different spatial arrangement of the substitutions (the two Cl atoms) across the double bond.
- Draw the perspective formulae for the two isomers and label them with their correct designations.
- What type of isomerism do these represent?
- How many chiral centers exist in this molecule?
- Make a model of its mirror image and draw its Fischer projection.
- What type of isomerism is represented by these mirror images?
- Construct a model of a third isomer that is not a mirror image of the two above.