16: Qualitative Analysis of Everyday Chemicals (Experiment)
- Page ID
- 95883
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- The physical and chemical properties of ten common chemicals will be observed in this experiment. Some of these properties will be used to identify five unknowns. Everyday chemicals are used for this analysis to emphasize that chemistry is involved in many aspects of our daily lives.
This lab introduces qualitative analysis, the area of chemistry concerned with the identification of substances by their physical and chemical properties. Identifying unknown substances is an important part of chemistry, with applications in fields such as medicine and environmental chemistry. Materials can be characterized by observing their physical and chemical properties and/or by instrumental methods. Since identification of substances by their typical reactions can sometimes be relatively easy, quick and inexpensive compared to instrumental methods, it is frequently the method of choice.
In this lab some simple characteristic reactions of ten common everyday chemicals will be observed. The common names, systematic names, and formulae of these chemicals are listed in the table below.
|
Common names |
Systematic names |
Formulae |
|---|---|---|
|
Photographic fixer |
Sodium thiosulfate pentahydrate |
\(\ce{Na2S2O3*5H2O}\) |
|
Baking soda |
Sodium bicarbonate |
\(\ce{NaHCO3}\) |
|
Washing soda |
Sodium carbonate |
\(\ce{Na2CO3}\) |
|
Table salt |
Sodium chloride |
\(\ce{NaCl}\) |
|
Sugar |
“Glucopyranosyl fructofuranoside” |
\(\ce{C12H22O11}\) |
|
Epsom salt |
Magnesium sulfate heptahydrate |
\(\ce{MgSO4*7H2O}\) |
|
Alum |
Ammonium aluminum sulfate dodecahydrate |
\(\ce{NH4Al(SO4)2*12H2O}\) |
|
Chalk |
Calcium carbonate |
\(\ce{CaCO3}\) |
|
Cornstarch |
“a polymer of glucose” |
\(\ce{(C6H10O5)_x}\) |
|
Silica gel (sand) |
Silicon dioxide |
\(\ce{SiO2}\) |
The physical and chemical changes that will be used to identify these everyday chemicals are described below. Examples and net ionic equations for some of the reactions are also supplied.
Solubility
In general, polar solutes dissolve in polar solvents and nonpolar solutes dissolve in nonpolar solvents. This phenomenon is commonly described as “like-dissolves-like”. Water is the most common solvent for inorganic compounds. Water is a polar compound and thus readily dissolves polar compounds, as well as many ionic compounds. Solubility rules in textbooks or handbooks contain general information about the solubility of ionic compounds in water.
When substances dissolve in water, the process can be exothermic (giving off heat and thus making the container warmer) or endothermic (absorbing heat and thus making the container colder). For example, the dissolution of Epsom salt is an endothermic reaction:
\[\ce{MgSO4*7H2O (s) + heat ->[\ce{H2O}] Mg^{2+} (aq) + SO4^{2-} (aq)}\]
Precipitation Reactions
Some soluble substances form a precipitate with the addition of a precipitating reagent. The appearance of the precipitate can provide a clue regarding the identity of a substance. For example, Epsom salt forms a milky precipitate when it reacts with ammonium hydroxide, while alum forms a gelatinous precipitate when it reacts with ammonium hydroxide. The net ionic equations for these two reactions are as follows:
\[\ce{Mg^{2+} (aq) + 2 OH^{1-} (aq) -> Mg(OH)2 (s)}\]
\[\ce{Al^{3+} (aq) + 3 OH^{1-} (aq) -> Al(OH)3 (s)}\]
Formation of a precipitate is often used to confirm the presence of specific ions in solution. For example to confirm the presence of chloride ions, silver ions are added to form silver chloride precipitate.
Acidity and Basicity
Substances that are acids produce an excess of hydrogen ions in water, resulting in a solution pH less than seven. Substances that are bases produce an excess of hydroxide ions in water, resulting in a solution pH greater than seven. The pH of solutions can be estimated using different indicators. An indicator is a weak organic acid which changes color depending on ts pH. An indicator that can measure large range of pH is called a universal indicator.
Acid-Base Reactions
Some of the household chemicals in this experiment are weak bases that contain carbonate or bicarbonate ions. These weak bases will react readily with acids such as acetic or hydrochloric acid producing carbon dioxide gas:
\[\ce{CO3^{2-} (aq) + 2H^{1+} (aq) -> CO2(g) + H2O(l)}\]
\[\ce{HCO3^{1-} (aq) + H^{1+} (aq) -> CO2 (g) + H2O(l)}\]
Electrical Conductivity
Substances that produce ions in solution are called electrolytes. The ions in solution conduct electric current because they are charged particles that are free to move about in the solution. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Strong electrolytes include soluble ionic compounds and strong acids. For example, table salt is a strong electrolyte (dissociates completely):
\[\ce{NaCl (s) -> Na^{1+} (aq) + Cl^{1-} (aq)}\]
Oxidation-Reduction Reactions
Oxidation-reduction reactions involve the transfer of electrons from an atom of one reagent to an atom of another reagent. The reagent that loses the electrons is called the reducing agent and the one that gains electrons is called the oxidizing agent. For example, photographic fixer turns brown iodine (\(\ce{I2}\)) solution colorless because the iodine gains electrons and becomes colorless iodide (\(\ce{I^{1-}}\)) ions, while the sulfur in the \(\ce{S2O3^{2-}}\) loses electrons and becomes \(\ce{S4O6^{2-}}\).
\[\ce{I2 (aq) + 2 S2O3^{2-} (aq) -> 2 I^{1-} (aq) + S4O6^{2-} (aq)}\]
Starch-Iodine Reaction
There are some reactions that only apply to one substance, or very small groups of substances. When these reactions occur they give a unique result and therefore are used to identify specific unknown substances. An example is the reaction of starch with iodine. When drops of iodine solution are added to starch, a characteristic blue-black complex is produced. This reaction is often used to confirm the presence of iodine.
Procedure
Chemicals and Equipment
Chemicals: Everyday chemicals, 1.0 M \(\ce{NH4OH}\), 1.0 M \(\ce{HC2H3O2}\), \(\ce{I2}\) solution.
Equipment: 10 large or medium test tubes, test tube rack, stirring rod, universal indicator paper and spot plates*
*obtain from the stockroom
Even though this lab deals with everyday chemicals, some of the substances are hazardous so never taste or touch any chemicals in the lab. Handle all solid chemicals with spatulas or spoons, and never use your finger to stopper test tubes.
Part A: Determining the Solubility of Everyday Chemicals in Water
Use the following steps to determine the solubility of each chemical in water.
- Use your spatula (or spoon) to obtain a very small amount (a half a pea-size or less) of each of the everyday chemicals and put each in separate large test tubes.
- Add about 10 mL of water to each test tube and mix well.
- Record which of the everyday chemicals are soluble and which are not. When a substance dissolves, the resulting solution will be clear (not cloudy).
Part B: If the Everyday Chemical is Soluble in Water
If a chemical is determined to be soluble in Step 1, carry out the following procedure.
- First, make up a fresh, more concentrated solution of each soluble substance by dissolving a full scoop of the chemical in 10 mL of water in a large (and clean) test tube. While mixing each, be sure to hold the test tubes and feel for temperature changes. Record which grow noticeably cooler or hotter upon dissolving. Save these solutions as your stock solutions and use a small, fresh portion of each for each test below.
- Add 20 drops (about 1 mL) of each solution to separate small test tubes. Add 5-10 drops of ammonium hydroxide solution (aqueous ammonia) to each. Observe which solutions form a precipitate. Record the type of precipitate (gelatinous or milky) formed.
- If the substances do not react with ammonia, estimate the pH of a fresh sample of each solution by placing one or two drops of each on pieces of universal indicator paper. Record the pH of each, and indicate whether the solutions tested are acidic, basic or neutral.
- For the substance(s) that are not basic, place about 10 drops of fresh solution of each in spot plate wells. Add 5 -10 drops of iodine solution (\(\ce{I2}\)) and record which ones decolorize the brownish iodine.
- For the substance(s) that do not decolorize iodine, test for conductivity by mixing two scoops (about 0.5 g) of each solid with about 40 mL of water in separate 150 mL beakers. Stir well, then test for conductivity by inserting the electrodes of the conductivity apparatus into the solution in the beaker.
Part C: If the Everyday Chemical is NOT Soluble in Water
If a chemical is determined to not be soluble in Step 1, carry out the following procedure.
- Place a small scoop of each solid chemical in separate spot plate wells, then peform the tests below. Use fresh samples for each test.
- Add 10 drops of vinegar (acetic acid) and record which substances produce gas.
- Add 2-3 drops of iodine solution and record which substance produces a blue-black complex.
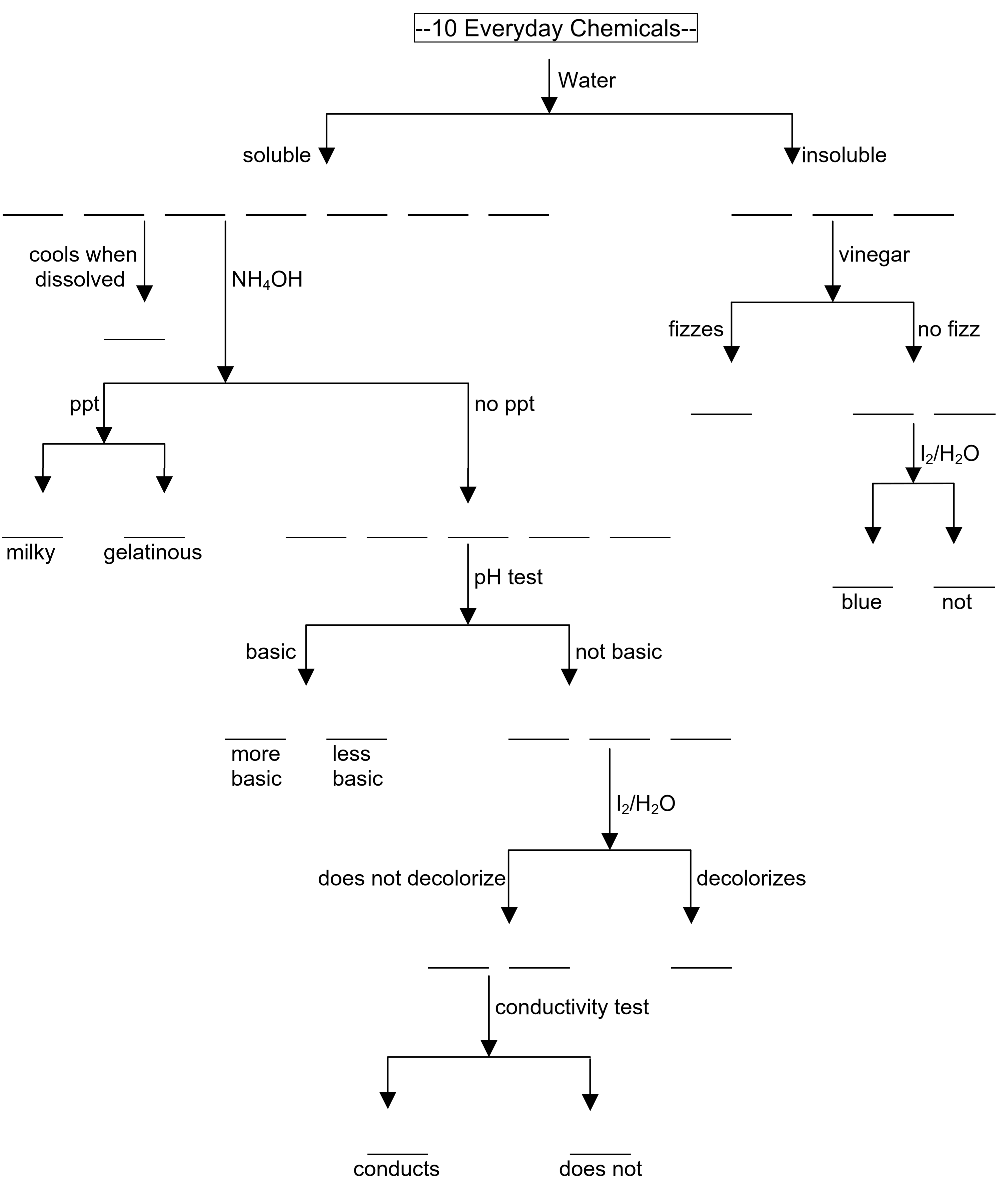
- Using your results from Steps 1 – 3, complete the flow chart in the data section of your Report form and have your instructor check it.
Part D: Analysis of Unknown Chemicals
Obtain samples of five different unknown chemicals. Write down the ID codes of your unknowns on your Report form.
Devise a scheme for determining the identity of the unknowns. If you wish, you may use the same scheme that you have used for the knowns, or you may try an alternate approach. Record all the reagents used, your observations, and your conclusions about the identity of each unknown in the table provided.
Pre-laboratory Assignment: Qualitative Analysis of Everyday Chemicals
- Look up a common use for each of the following everyday chemicals. You may use textbooks, the internet, or encyclopedias for your sources.
- Epsom salt
- Alum
- Corn starch
- Photographic fixer
- Baking soda
- Sodium chloride
- Which of the above chemicals are soluble in water? If needed refer to solubility rules guides in your textbook.
- Which solution is a better conductor of electric current, 1 M \(\ce{HC2H3O2}\) or 1 M \(\ce{HCl}\)? Explain.
- Suppose an everyday chemical reacts with vinegar. Is the substance more likely to be an acid or a base?
- Write the molecular equation and the net ionic equation for the reaction that occurs between aqueous sodium chloride and aqueous silver nitrate.
- Circle the substances given below that produce a basic solution (pH > 7) when dissolved in water?
- \(\ce{NaCl}\)
- \(\ce{KOH}\)
- Sucrose
- \(\ce{NH3}\)
- Vinegar
- Provide one simple chemical test that can distinguish between \(\ce{NaCl}\) and \(\ce{Na2CO3}\).
- Identify the oxidizing and the reducing agent for the following reaction.
\[\ce{I2 (aq) + 2 S2O3^{2-} (aq) -> 2 I^{1-} (aq) + S4O6^{2-} (aq)}\]
Lab Report: Qualitative Analysis of Everyday Chemicals Analysis of Everyday Chemicals
Analysis of Everyday Chemicals
Record your results from procedural steps 1-3 in the table below. If a test is not performed on a substance, leave that space blank, or write N/A (not applicable).
|
Household chemicals |
Soluble in \(\ce{H2O}\) |
Forms ppt with \(\ce{NH3}\) |
pH |
Reacts with Vinegar |
Reacts with iodine |
Conducts e- current |
|---|---|---|---|---|---|---|
|
Table salt |
||||||
|
Sugar |
||||||
|
Epsom salt |
||||||
|
Alum |
||||||
|
Photographic fixer |
||||||
|
Cornstarch |
||||||
|
Aquarium sand |
||||||
|
Chalk |
||||||
|
Baking soda |
||||||
|
Washing soda |
Flow Chart
Use your results from the preceding table and the following legend to complete the flow chart.
|
Name |
photo fixer |
baking soda |
washing soda |
table salt |
sugar |
epsom salt |
alum |
corn starch |
sand |
chalk |
|---|---|---|---|---|---|---|---|---|---|---|
|
Legend |
pf |
bs |
ws |
ts |
su |
es |
al |
cs |
sa |
ch |
Analysis of an Unknown Chemicals
Record all the reagents used, your observations, and your conclusions about the identity of each unknown analyzed in the table below.
|
Unknown # |
Test reagent(s) used |
Observations |
Identity of Unknown |
|---|---|---|---|