Umami and Protein
- Page ID
- 50931
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ramen. Mac'n cheese. Takeout pizza. A college student's culinary habits are confessedly not quite the healthiest. One food that enjoyed (and still be enjoying) its unhealthy 15 minutes of fame throughout the entirety is, yes, bacon. Bacon festivals offering every dish from bacon maple doughnuts to bacon ice cream[1] have been held across the country, inspiring a sort of "Bacon Nation" cult that centers around the pork-derived grease product.
Figure \(\PageIndex{1}\) Raw bacon, ready to be cooked and devoured.
What makes bacon and other foods and seasonings like barbecue sauce and tortilla chips so addicting and satisfying to the diet-shunning public? The answer lies in the relatively unknown fifth cousin of the common taste classifications (sour, sweet, bitter, salty), which is borrowed from the Japanese language: umami. In English, it may be translated to roughly "savory" or "brothy" - a good sign for the taste buds. The receptor cells in the tongue recognize certain compounds in foods, which are formally classified as one of the five tastes mentioned above.
You may already be able to guess certain compounds that are responsible for each of these tastes; simple carbohydrates like sucrose are sweet, and saltiness is mostly due to sodium chloride. Umami might be a harder case to find examples for; what exactly is it in bacon that contributes such an addicting flavor? The general answer may seem initially alarming, depending on how knowledgeable about food chemistry you are.
Chemistry of Umami
Glutamic acid, one of the 20 proteins essential to human life, is responsible for the taste of umami, but as it does deprotenate in solution, it is difficult to isolate from the foods it naturally occurs in, such as cheese and soy sauce. Charged particles also cannot exist on their own and will form a stabilizing bond if given the opportunity. In order to artificially add glutamic acid to other foods, glutamic acid needs to bond to an acceptable cation to form a salt, which is easily transportable in a solid state and can dissolve in solution to release the glutamic acid ion. These salts are called glutamates, and are differentiated by the particular cation used in synthesizing the salt.
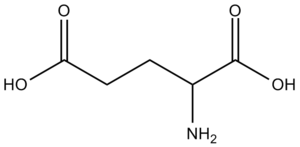
Example \(\PageIndex{1}\): Glutamic Acid
The image of glutamic acid given is a neutral compound before deprotenation. Identify which hydrogen is deprotenated in order to give the glutamate anion.
Solution:
Glutamic acid is a carboxylic acid, so one of the (-RCOOH) groups releases its proton in solution, as opposed to the amine group. It is difficult to decide which carboxylic acid group will preferably deprotenate. The group on the right is part of the amide backbone that all proteins contain; in an amino acid, this (-RCOOH) group would participate in a condensation reaction with the amine group of another amino acid to create a protein chain.
The carboxylic acid group on the left is part of an "R" group, which differs between each amino acid; most amino acids do not have another carboxylic acid group in their R group. Therefore, we know that the carboxylic acid group in the amide group will always deprotenate first when given the chance, as we know that all amino acids can participate in a condensation. The deprotenation of glutamic acid's R group is a secondary occurrence.
Glutamate Salts
The most common cation used to isolate glutamate as a salt is sodium, Na+. The resulting salt is then called..... ready? Monosodium glumatate, commonly known as the dreaded acronym MSG. MSG was commonly added to many preserved foods for its savory umami taste. However, health concerns have prompted its removal from many products, though cases of serious MSG intolerance or symptoms have not been found reliable[2]. Though advertised as being absent in such places as Chinese restaurants and products such as ramen, food companies have found a clever way to circumvent the public's prevailing wariness of MSG.

Figure \(\PageIndex{2}\) Ramen noodles.
The important thing to realize is that it is not so much the "MS", or the sodium ion, that matters in the chemical properties of MSG, but the "G", glutamate, as it is ultimately responsible for the taste. Thus, a product advertised as containing "No MSG" may very well contain other sources of glutamate, such as other glutamate salts, or free glutamate, which is metabolized differently in proteins. MSG is simply the most common salt of glutamate used in industry and thus is stuck with the unhealthy stigma.
Example \(\PageIndex{2}\) : Glutamate Salts
a) Name another possible glutamate salt by choosing another cation besides sodium.
b) Name another possible glutamate salt, but this time, retain sodium. (Hint: consider the previous example and how sodium reacts with the glutamate molecule.)
Solution:
a) Any of the Group I metals (alkali metals) is acceptable, as a charge of +1 is needed to neutralize the charge of -1 left on an O atom of a carboxylic acid group after it is deprotenated. One example would be K+, giving monopotassium glutamate. The larger metals of Group I are generally not used in organic chemistry and food chemistry due to their scarcity and the size of the atom.
b) The clue given references the secondary deprotenation of the carboxylic acid group in the R-chain of glutamic acid. A sodium ion can also form an ionic bond with the negatively charged O atom after it is deprotenated. In the previous example, we said that (-RCOOH) in the amide group would be deprotenated first. Therefore, the resulting compound would have both (-RCOOH) groups deprotenated and ionically bonded to a sodium atom, giving disodium glutamate, or DSG.
From ChemPRIME: 20.10: Proteins
References
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


