Molecular Gastronomy: Cooking in a Vacuum
- Page ID
- 49944
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A new approach to cuisine and cooking called Molecular Gastronomy is gaining in popularity. Originated in 1992 by French physical chemist Hervé This (pronounced Tees) and Hungarian physicist Nicholas Kurti[1], its practitioners create unusual dishes by using the tools and molecular understanding of chemistry.
In his book "Molecular Gastronomy"[2], H. This describes "Cooking in a Vacuum" (chapter 83), where he uses a simple vacuum pump commonly found in laboratories, but never before used in professional or home cookery. The venturi or "aspirator" pump attaches to a faucet, and creates a vacuum when the water runs strongly. The sidearm of the pump is attached to the hose shown in the picture of the filtering apparatus below, to draw a liquid through the filter, leaving solids on its surface.


Beef or vegetable stock is made by simmering solid ingredients in water for hours. The solids are traditionally removed to "clarify" the stock, by filtering it through a cloth. Sometimes eggwhite is added first, which traps much of the solid as it solidifies, making filtration easier. But the eggwhite also removes flavor. H. This says "Imagine going to the trouble of cooking a stock for several hours and then having to re-enrich it because cooking has impoverished it." Instead, he uses the vacuum filter apparatus above to remove the solids, even producing a completely clear stock. The solids do not dissolve because they are made up of close-packed atoms or large molecules, held together with strong interatomic or intermolecular bonds. On a molecular level, they would look like the figure below, where each dot might represent an atom (in a solid iron skillet, for example) or molecules, like the actin molecules that help create strong muscle fibers[3], or much larger cellulose molecules in vegetable skins. The molecules in a solid vibrate and twist to some extent in position, but don't move relative to one another.

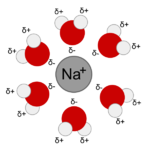
The liquid water of the stock solution dissolves various molecules in the meat because molecules in a liquid can move around, as shown in the figure below. The molecules surrounding small molecules near the surface of the solid, bonding to them, and extracting them into the bulk liquid water. Ions like sodium (Na+ (along with negative ions) are also extracted from the solid by the liquid water, as shown in the figure below. Because the particles in solids and liquids are in contact with each other, they have similar densities, as we have seen, varying from a little below 1 g/ml to around 10 g/ml (with a few solids higher).

Figure \(\PageIndex{6}\) Atoms or molecules in typical liquid
The dissolved ions and molecules are much too small to be removed by filtration, so they pass through the filter, as the "stock".
Some of the small molecules in the stock are bonded weakly enough to the water so that they evaporate, creating the aroma of the stock that reaches our noses. Gas molecules have very weak intermolecular forces, so they separate, as shown in the figure below, and fill any space available to them. The movement of the molecules (around 100 meters per second, depending on their mass) creates pressure on their container. Because the molecules of a gas are separated by large distances, they have densities only around 1/1000 as large as liquids and solids (air has a density of about 1.3 g/L or 0.0013 g/ml).
From Meringue to Soufflé
But H. This then reports that Nicholas Kurti thought about what else the aspirator pump might be used for, and he imagined making lighter meringes with them. Meringes are made by whipping air into a mixture of sugar and eggwhites, then cooking the mixture on low heat. Coagulation of the proteins makes the walls of air bubbles stable, creating a stiff foam when water evaporates. What if the meringue were made by removing the water by evaporation in a vacuum, rather than with heat? This would make an even lighter foam, because the air bubbles would expand in the vacuum. But the result was something like an aerogel, so light that there isn't anything to bite into.
What if a more substantial liquid were used, like the flour, butter, milk, and eggs used to make a soufflé or cream puff? The soufflé batter expands in the vacuum chamber as the air bubbles grow, but the bubbles collape as soon as the soufflé is removed from the chamber. They're working on cooking it under vacuum.
As you can begin to imagine, applying chemical principles to food preparation can lead to some very interesting results!
You may have noticed that although our microscopic model can explain many of the properties of solid, liquid, and gaseous materials, it isn't easy to explain all of them. The flavor of the stock is requires a complex explanation on the atomic or molecular level, involving the interactions of molecules with odor and taste receptors in our nose and tongue that aren't fully understood. It is perfectly reasonable to extend the atomic theory so that it can account for these facts. In the current section on Atoms, Molecules and Chemical Reactions as well as Using Chemical Equations in Calculations we shall discuss in more detail those facts that require only a simple atomic theory for their interpretation. Many of the subsequent sections will describe extensions of the atomic theory that allow interpretations of far more observations.
From ChemPRIME: 2.1: Macroscopic Properties and Microscopic Models
References
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


