Chemistry in Foods
- Page ID
- 50670
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)When we think about choosing healthy foods, we often are inclined to avoid ones with "chemicals". But after a little more thought, we might wonder about how many toxic "chemicals" are actually derived from plants, or if there is really a difference between the substances found in plants and synthetic chemicals added by food manufacturers. This is a subtle question; even an excess of the essential vitamin E may cause diabetes [1].
Many of the most toxic chemicals are found in plants. An example of a toxic plant chemical is the oxalic acid (or calcium oxalate, see Figure below), found in rhubarb, spinach, agave, kiwi, and several other fruits and vegetables. Spinach and rhubarb are toxic (in very high doses) because the oxalate inhibits cellular energy production and forms crystals in the kidneys (kidney stones), sometimes causing renal failure. Since oxalate bonds to calcium (Ca) strongly, high oxalate levels can lead to calcium deficiency. A fatal dose of oxalate is 1500 mg, and spinach has 600 mg/100 g (dry weight), peanuts 150 mg/100 g, rhubarb 500 mg/100g (much higher in leaves), and a cup of tea 50 mg.[2]
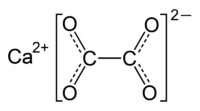
Calcium oxalate molecular structure

Calcium oxalate crystals in urine
Because of its focus on the molecular level, chemistry can reveal connections that we otherwise would not notice. For example, oxalate poisoning also results from injestion of antifreeze (ethylene glycol), which is sometimes used in suicide attempts [3], and occasionally ingested by animals because of its sweet taste. The structure of ethylene glycol is similar to oxalic acid, except one oxygen atom on each carbon (C) atom is replaced with two hydrogen atoms.
So chemistry may be the source of some substances in food that are undesirable, it is more often the source of information about what substances are present in foods, and how they affect our bodies. IWC replica Réplica de reloj Because oxalate bonds even more strongly to metals other than calcium (Ca), it is found in rust removers and industrial cleaners, and even in the cleanser "Bar Keeper's Friend" [4]. Some plants, like the houseplant dieffenbachia, actually contain the calcium oxalate crystals in another, needle-like form called "raphides", shown below. Contact with the plant's raphides can cause fairly severe skin irritation.[5]

Dieffenbachia houseplant
So how can a study of chemistry help us understand what is good for our bodies and what is bad?
The science of chemistry is concerned with the composition, properties, and structure of matter and with the ways in which substances can change from one form to another. Since anything that has mass and occupies space can be classified as matter, this means that chemistry is involved with almost everything in the universe. But this definition is too broad to be useful. Chemistry isn't the only science that deals with the composition and transformations of matter. Some matter is composed of cells, which transform by meiosis and other processes that biologists study. Matter is also composed of subatomic particles called leptons, which transform by processes like annihilation studied by physicists. Chemists are unique because they understand or explain everything, from our bodies to our universe, in terms of the properties of just over 100 kinds of atoms found in all matter and the amazing variety of molecules and other atomic-scale structures that are created by forming and breaking bonds between atoms. So chemistry is defined by its approach, not its subject matter. Chemistry explains or understands any subject in terms of the properties of atoms and molecules. Chemistry, in other words, is not just something that happens in laboratories. It is a unique perspective, or way of understanding, all that is around us, and even inside us.
Because of its focus on the atomic composition of substances, chemistry reveals the connections between the chemicals in "healthy" spinach and industrial cleaners!
Chemistry can be applied in places as diverse as the smallest bacterium, a field of ripening wheat, a modern manufacturing plant, the biospheres of planets such as Earth, the vast reaches of interstellar space, and even your eyes and brain as you read these words.
ChemPRIME recognizes that the chemical perspective can add to our understanding of anything that catches our interest, like the food we eat. Our goal will be to add another dimension--understanding in terms of the properties of atoms and molecules and how they interact--to many subjects, thereby making it clear how the study of chemistry is of importance to a wide variety of people. Biologists, for example, have examined smaller and smaller organisms, cells, and cell components, until, in the study of viruses and genes, they joined forces with chemists who were interested in larger and larger molecules. The result was a new inter-disciplinary field called molecular biology, and a reinforcement of the idea that living organisms are complicated, highly organized chemical systems. Chemists interact in similar ways with scientists in areas such as chemical physics, geochemistry, pharmacology, toxicology, ecology, meteorology, oceanography, and many others. Current practice in these fields is such that a person lacking basic chemical knowledge is at a severe disadvantage, because the perspective of the molecular level has become so important.
Chemistry also underlies a great deal of modern food technology, including toxin removal, preservation, allowing safe preservation and distribution by de-activating spoilage and pathogenic micro-organisms, and increasing food consistency. These benefits are not without costs. A consumer must be knowledgeable to recognize when processed (even home-processed) food may be inferior. Vitamin C, for example, is destroyed by heat and therefore canned fruits have a lower content of vitamin C than fresh ones. On the other hand, processing can enhance vitamin and mineral content of foods. Processed foods may include food additives which may have unanticipated negative effects. The antioxidants BHA and BHT have been found to retard spoilage, thus avoiding waste and some disease. Some studies have linked them (at high doses) to cancer in some laboratory animals [6], but other studies have actually shown that BHA and BHT actually preserve health and extend life expectancy (in mice) [7] and it is sold as a health food supplement [8]
Control of the food industry is political, at least in part, and requires some chemical knowledge on the part of consumers as well as their elected representatives. At the very least, a citizen needs to be able to distinguish valid and invalid arguments put forward by scientific “experts” regarding such issues. Is the risk posed by fumigants higher than the risk caused by aflatoxin[9], a chemical toxin found naturally in peanuts and other grains, and possibly the strongest carcinogen known?

The aflatoxin molecule
Aflatoxin-producing members of Aspergillus are common and widespread in nature. They can colonize and contaminate grain before harvest or during storage, but they are easily controlled by fumigation.
Given the universality of chemistry, its central role among the sciences, and its importance in modern life, how is it possible to learn much about it in a short time? If everything has a chemical aspect, because atoms and molecules can aid in understanding everything, is the field of chemistry so broad and all-encompassing that one cannot master enough to make its study worth-while? We think the answer to this second question is a resounding no! This entire book has been designed to help you learn a good deal of chemistry in a short time. If it is successful, the first question will have been answered as well.
References
- ↑ Atul Butte, PLoS One May 20, 2010,
- ↑ Emsley, J. “Molecules at an Exhibition,” Oxford U. Press, 1998
- ↑ http://informahealthcare.com/doi/pdf/10.1080/15563650701419011
- ↑ en.Wikipedia.org/wiki/Bar_Keepers_Friend
- ↑ en.Wikipedia.org/wiki/Dieffenbachia
- ↑ en.Wikipedia.org/wiki/Butylated_hydroxyanisole#Carcinogenicity
- ↑ Silverstein, A. and V. Silverstein, The Chemicals We Eat and Drink, Follett publishing Co., Chicago, 1973, p. 45
- ↑ en.Wikipedia.org/wiki/Butylated_hydroxytoluene#Controversy
- ↑ en.Wikipedia.org/wiki/Aflatoxin
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


