Bio-diesel and Thermodynamics
- Page ID
- 50907
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exothermic reactions: Combustion of bio-diesel extracted from algae
Exothermic reactions are the reactions that release energy. It is typically depicted in the following form:
A+ B ---->C+ energy (Here A and B are reactants and C denotes the product)
Equation 1: Exothermic reaction
The quantity of the energy released in this reaction is calculated using bond energies released and absorbed in a particular reaction. Whenever any chemical reaction occurs, reactant bonds break and energy is released. Then products form new bonds and energy is consumed. If the energy released is more than the energy required, then it qualifies as exothermic reaction, otherwise it is an endothermic reaction. An example of exothermic reaction would be - combustion of methane, as explained below.
Before learning about combustion, let’s establish enthalpy of reaction. The standard bond enthalpy (Ho) is the energy required to break one mole of the reactant’s bonds and can be used to calculate overall heat of a reaction, as described in the following equation:
Ho (heat of reaction) = sum of the bond enthalpies for bonds broken in the reaction - sum of the bond enthalpies of the bonds formed in the reaction.
Equation 2: Enthalpy of Reaction
Now, combustion is an exothermic reaction in which heat is released when a substance is burned in the presence of oxygen. Following is an example of combustion reaction (see methane combustion) below:
Methane + 2 oxygen ==> carbon dioxide + 2 water + energy
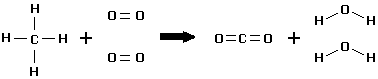
Given the bond energy of reactants and products (See table 1.1), one can calculate the heat of reaction.
Table 1.1
|
Bonds |
Energy |
|
C-H single bond |
413 KJ/mol |
|
O=O double bond |
495KJ/mol |
|
H-O single bond |
463 KJ/mol |
|
C=O double bond |
799 KJ/mol |
Calculations of the reactant side compounds :
Bonds broken and heat energy absorbed from surroundings, endothermic change:
(4 x C-H) + 2 x (1 x O=O) = (4 x 413) + 2 x (1 x 495) = 2642 kJ/mol taken in.
Calculations of the product side compounds:
Bonds formed and heat energy released and given out to surroundings, exothermic change: (2 x C=O) + 2 x (2 x O-H) = (2 x 799) + 2 x (2 x 463) = 3450 given out.
By the equation 1: Energy released = 2642-3450 = -808 kJ/mol
Now you see how 808 KJ/mol of energy is released when one mole of methane is combusted in the presence of oxygen. Energy released is depicted by the negative sign.
This concept can be well understood using the relative energies of the product and reactant, as seen in the energy profile diagrams drawn below:
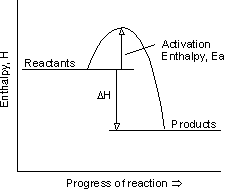
Figure 1: Energy of reactant is more than the energy of product. Energy is released when molecules collide after attaining the Ea (activation enthalpy)
Combustion reactions always involve molecular oxygen (O2). Usually, when organic molecules break in the presence of oxygen, the products are mostly carbon dioxide and water (as well as heat).
Organic Compound (C, H & O) + O2------> CO2 + H2O + Heat
Note: Not all compounds on combustion release CO2 and water
For example: Mg + O2 ------> MgO + heat
== = Biochemistry of algae = ==
As you have seen above, energy is produced during the combustion of organic molecules. One such molecule of bio-diesel comes from green energy source - algae. Actually, the substance extracted from the algae is a glyceride which is trans-esterified (see reaction 1.2) into the biodiesel.
Transesterification is the process in organic chemistry that is employed to convert one ester into another, in the presence of a strong base (NaOH or KOH) [5]. Chemically, biodiesel can be a methyl orethyl ester depending upon the type of alcohol used in trans-esterification process [6].
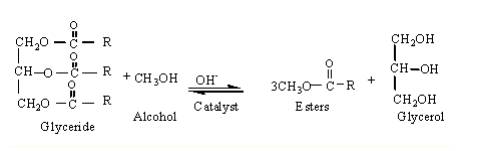
Reaction 1.2 above shows reactions taking place where one molecule of triglyceride (from algal extract) reacts with three molecules of methanol to produce three molecules of methyl ester (bio-diesel), while one molecule of glycerol separates.
Finally, this bio-diesel molecule produced in the reaction is used to produce energy through combustion in machines. Given the bond energies and chemical structure of the biodiesel, one can calculate the amount of energy released on the complete combustion of one molecule of biodiesel (methyl ester).
Structure of Biodiesel molecule [7]:
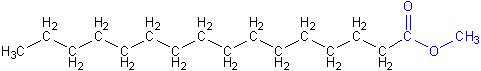
Average Bond Enthalpies of various bonds involved in the reaction:
Table 1.3:
|
C-H |
413 KJ/mol |
|
C-C |
348 KJ/mol |
|
C=O |
799 KJ/mol |
|
O=O |
495 KJ/mol |
|
C-O |
358 KJ/mol |
|
O-H |
463 KJ/mol |
Try calculations and find Heat of Combustion of Bio-diesel
Check your calculations here: Key
Step 1) Assess the number and types of bonds broken and formed in the balanced reaction equation. Use the molecular structure of various compounds involved in the reaction.
Step 2) Multiply the average bond energy (table 1.3) with the number of bonds
Step 3) Put the values in equation 2 (see the first paragraph)
Balanced reaction :
2 C17H30O2 + 47 O2 = 34 CO2 + 30 H2O + energy
For one mole of the biodiesel the reaction would be:
1 C17H30O2 + 23.5 O2 = 17 CO2 + 15 H2O + energy
Energy of reactant:
34 (C-H) + 1 (O=C) +1 (C-O) + 15 C-C] + 23.5 (O=O)
34(413) + 799 +358 + 15 (348) + 23.5 (495) = 32 051.5KJ/mol
Energy of the product:
17 *2 *(C=O) +15 (2) (H-O)
17 (2 * 799) + 15 (2 * 463) = 41 056KJ/mol
By equation 2:
Energy released = Energy of bonds broken - Energy of bonds formed = 32051-41056 = -4 502.5 KJ/mol (negative sign demonstrates the exothermic reaction).
This energy can be potentially released with complete combustion of biofuels. Research is on its way to achieve maximum energy of combustion of biofuels through consistent changes in the engine design.
From ChemPRIME: 15.0: Prelude to Thermodynamics
References
[1] http://www.docbrown.info/page03/3_51energy.htm
[2] www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/react2.htm
[3] www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/react2.htm
[3]http://www.saskschools.ca/curr_content/chem30_05/appendix/tables_charts/bond_enthalpies.pdf
[4]"http://www.google.ca/imgres?imgurl=http://www.avogadro.co.uk/h_and_s/hprofile.gif&imgrefurl=http://www.avogadro.co.uk/h_and_s/enthalpy.htm&h=189&w=225&sz=2&tbnid=Q8oUgj7PxbetMM:&tbnh=91&tbnw=108&prev=/search%253Fq%253Denergy%252Bexothermic%252Bprofile%252Bdiagram%2526tbm%253Disch%2526tbo%253Du&zoom=1&q=energy+exothermic+profile+diagram&hl=en&usg=__CDQAgX2u9Cas8sitHAEZa2xgVHc=&sa=X&ei=RFkFToGFJvSOsAKf_snwDQ&ved=0CB8Q9QEwAg">http://www.google.ca/imgres?imgurl=http://www.avogadro.co.uk/h_and_s/hprofile.gif&imgrefurl=http://www.avogadro.co.uk/h_and_s/enthalpy.htm&h=189&w=225&sz=2&tbnid=Q8oUgj7PxbetMM:&tbnh=91&tbnw=108&prev=/search%3Fq%3Denergy%2Bexothermic%2Bprofile%2Bdiagram%26tbm%3Disch%26tbo%3Du&zoom=1&q=energy+exothermic+profile+diagram&hl=en&usg=__CDQAgX2u9Cas8sitHAEZa2xgVHc=&sa=X&ei=RFkFToGFJvSOsAKf_snwDQ&ved=0CB8Q9QEwAg
[5]. Robert B. Levine, Tanawan Pinnarat, and Phillip E. Savage*
2010, "Biodiesel Production from Wet Algal Biomass through in Situ Lipid Hydrolysis
and Supercritical Transesterification"Energy Fuels:ACS publication, 2010, 24 (9), pp 5235–5243
[6] www.ag.ndsu.edu/pubs/ageng/machine/ae1240w.htm
[7] http://www.goshen.edu/chemistry/biodiesel/chemistry-of/ </div>
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


