Acids and Bases
- Page ID
- 50869
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The most straight-forward examples involving acids and bases deal with strong acids and bases. Strong acids, like HCl or HNO3, are such good proton donors that none of their own molecules can remain in aqueous solution. All HCl molecules, for example, transfer their protons to H2O molecules, and so the solution contains only H3O+(aq) and Cl–(aq) ions. Similarly, the ions of strong bases, like BaO or NaH, are such good proton acceptors that they cannot remain in aqueous solution. All O2– ions, for example, are converted to OH– ions by accepting protons from H2O molecules, and the H2O molecules are also converted to OH–. Therefore a solution of BaO contains only Ba2+(aq) and OH–(aq) ions.
Table 1 lists molecules and ions which act as strong acids and bases in aqueous solution. In addition to those which react completely with H2O to form H3O+ and OH–, any compound which itself contains these ions will serve as a strong acid or base. Note that the strength of an acid refers only to its ability to donate protons to H2O molecules and the strength of a base to its ability to accept protons from H2O molecules. The acidity or basicity of a solution, on the other hand, depends on the concentration as well as the strength of the dissolved acid or base.
Table \(\PageIndex{1}\) Species Which Are Strong Acids and Bases in Aqueous Solution.
| Strong Acids | Strong Bases |
|
H3O+ (Only a few compounds like H3OCl and H3ONO3 are known to contain hydronium ions.) |
OH– [Only LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2 and Ba(OH)2 are sufficiently soluble to produce large concentrations of OH–(aq).] |
|
HCl, HBr, HI HClO4 |
O2– (Li2O, Na2O, K2O, Rb2O, Cs2O, CaO, SrO, and BaO are soluble |
| HNO3, H2SO4, HClO3 | H–, S2–, NH2–, N3–, P3– |
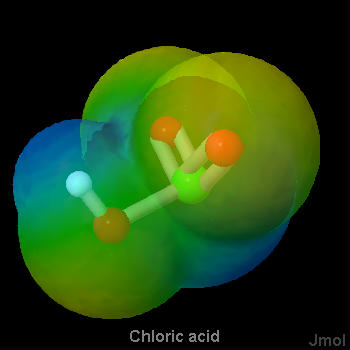
As a general rule, strong proton donors are molecules in which a hydrogen is attached to a rather electronegative atom, such as oxygen or a halogen. Considerable electron density is shifted away from hydrogen in such a molecule, making it possible for hydrogen ions to depart without taking along any electrons. The strong acids in Table 1 fit this rule nicely. They are either hydrogen halides (HCl, HBr, HI) or oxyacids (whose general formula is HnXOm). Below are a set of lewis structures for some of the strong oxyacids. For each lewis structure, there is also a 3D Jmol model which shows the electrostatic potential of along the Van der Waals surface of the molecule. It can be seen that the acidic hydrogen atoms all show a distinct blue coloration, denoting partial positive charge, and that the oxygen connected to the acidic hydrogen shows an orange color which denotes a negative charge. This gives a sense of the polarization of the bond to the acidic hydrogen.
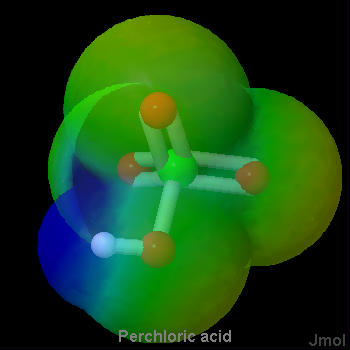
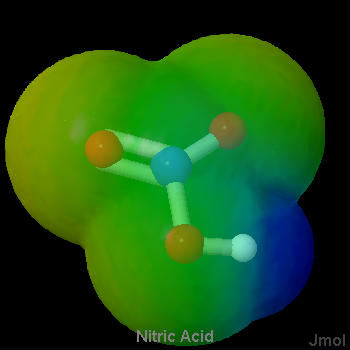
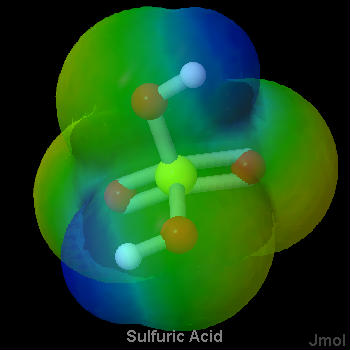
Figure \(\PageIndex{3}\) Sulfuric Acid
Chloric Acid
The Lewis structures indicate a proton bonded to oxygen in each of the oxyacids, hence their general name. Note that for a strong oxyacid the number of oxygens is always larger by two or more than the number of hydrogens. That is, in the general formula HnXOm, m ≥ n + 2.
The strength of a base depends on its ability to attract and hold a proton. Therefore bases often have negative charges, and they invariably have at least one lone pair of electrons which can form a coordinate covalent bond to a proton. The strong bases in Table 1 might be thought of as being derived from neutral molecules by successive removal of protons. For example, OH– can be obtained by removing H+ from H2O, and O2– can be obtained by removing H+ from OH–. When the strong bases are considered this way, it is not surprising that they are good proton acceptors.
Reactions in Aqueous Solutions in Environmental/Green Chemistry/Sustainability
Introduction
Most of the Earth is covered in water and our atmosphere contains a great deal of moisture. As such, most chemical reactions take place in the presence of water. Many of these reactions impact our environment and the lifeforms that inhabit our planet. Some of these reactions are due to the human impact on the planet. Dangerous gases such as sulfur dioxide and nitrogen dioxide are released from factories, automobiles, and volcanoes. Efforts are being made to minimize the negative effects human activity has had on the planet. One such devastating effect has been acid rain.
Acid Rain
Any precipitation (rain or snow) that has a pH lower than 5.7 is considered acid rain. It is caused by emissions of sulfur dioxide and nitrogen oxides; these emissions react with the water in our atmosphere to produce acids. Sulfur dioxide can be produced by volcanic eruptions and nitrogen dioxides by lightning strikes. Most of the harmful chemical are released by human activities. These chemicals travel up into the atmosphere, mix with rain clouds, and fall in the form of acid rain. The acid rain can cause corrosion of steel structures such as bridges, stone statues, and the peeling of paint. Acid rain also has a negative impact on trees, plants, and animals. It can significantly lower the pH of soil, rivers, lakes, and streams, thus negatively affecting organisms that prefer a more neutral or basic environment in which to thrive. Drinking water may also be affected. Acid rain is most prevalent on the East Coast of the United States where there is a higher density of industrial and population centers.
The following shows the reactions from sulfur dioxide, released from industry and volcanoes with water in our atmosphere:
- Sulfur dioxide is oxidized by reaction with the hydroxyl radical according to the following reactions:
\[\ce{SO2 + OH -> HOSO2}\nonumber\]
\[\ce{HOSO2 + O2 -> HO2 + SO3}\nonumber\]
- In the presence of water, sulfur trioxide becomes sulfuric acid:
\[\ce{SO3 + H2O -> H2SO4 (sulfuric acid)}\nonumber\]
\[\ce{NO2 + OH -> HNO3 (nitric acid)}\nonumber\]
- In a cloud, sulfur dioxide can by hydrolysed:
\[\ce{SO2 (g) + H2O <-> SO2 \cdot H2O}\nonumber\]
\[\ce{SO2 \cdot H2O <-> H+ + HSO3-}\nonumber\]
\[\ce{HSO3- <-> H+ + SO3 (2-)}\nonumber\]
- Acid rain can react with limestone as follows:
First, sulfur dioxide reacts with water to make sulfurous acid:
\[\ce{SO2 + H2O -> H2SO3}\nonumber\]
Second, sulfur trioxide reacts with water to make sulfuric acid (see graphic above):
\[\ce{SO3 + H2O -> H2SO4}\nonumber\]
Thirdly, aulfuric acid then further reacts with limestone in a neutralization reaction:
Limestone:
\[\ce{CaCO3 + H2SO4 -> CaSO4 + H2CO3}\nonumber\]
\[\ce{H2CO3 -> CO2 (g) + H2O}\nonumber\]
Finally, the calcium sulfate is soluble in water and hence the limestone dissolves and crumbles. As mentioned above, this can do considerable damage to bridges and statues. In some parts of the world, statues are covered during the winter to prevent acid rain (or snow) from causing further destruction to the artifact. Without the proper protection or reduction in the amount of acid rain produced, some artifacts may be lost forever. See http://www.epa.gov/acidrain/ for more information about acid rain.
From ChemPRIME: 11.0: Prelude to Aqueous Phase Reactions
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


