Biodegradable Plastics
- Page ID
- 50836
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)BIODEGRADABLE PLASTICS
Plastics have become a staple of modern life and are valued for their durability, strength, flexibility, light weight and low cost of production. They are versatile in that they carry our groceries home, hold our morning coffee, as well as control drug release and hold bones together. Plastics have become an environmentalists’ nightmare, however, because of their durability and dependence on oil. Many types of plastics persist in landfills and in the environment where they are inadvertently ingested by animals and are making their way up the food chain. Although recycling would be a good way to reduce the plastics in the landfills, currently “only 5% out of 1 trillion plastic bags, annually produced in the US alone, are being recycled” (Sivan, 2011).
One way to mitigate the trash dilemma is to design new biodegradable plastics. Several biodegradable plastics are currently on the market today. Biodegradable plastics have many suitable applications. They can be used in plastic grocery bags, food service and single serve disposable containers, personal care products, razors, brushes, applicators, cell phones, pens, and office supplies, agricultural plastics, mulch film, netting, sod stakes, erosion control netting, plant posts, plant clips and medical devices.
| Plastic Type | Use |
|---|---|
| Polylactic acid (PLA) |
Packaging and paper coatings; other possible markets include sustained release systems for pesticides and fertilizers, mulch films, and compost bags |
| Polyglycolic acid (PGA) |
Specialized applications; controlled drug releases; implantable composites; bone fixation parts |
| Polycaprolactone (PCL) |
Long-term items; mulch and other agricultural films; fibers containing herbicides to control aquatic weeds; seedling containers; slow release systems for drugs |
| Polyhydroxybutyrate (PHB) |
Products like bottles, bags, wrapping film and disposable diapers, as a material for tissue engineering scaffolds and for controlled drug release carriers |
| Polyhydroxyvalerate (PHBV) |
Films and paper coatings; other possible markets include biomedical applications, therapeutic delivery of worm medicine for cattle, and sustained release systems for pharmaceutical drugs and insecticides |
| Polyvinyl alcohol (PHV) |
Packaging and bagging applications which dissolve in water to release products such as laundry detergent, pesticides, and hospital washables |
|
Polyvinyl acetate |
Adhesives, the packaging applications include boxboard manufacture, paper bags, paper lamination |
Table found in (Shah et al., 2008)
(www.envis-icpe.com, Plastics recycling-Economic and Ecological
Options. ICPE 2006;4(4):1–12).
en.Wikipedia.org/w/index.php?title=Polyhydroxybutyrate&oldid=
189442759. Accessed February 14, 2008.
www.chemquest.com/store/polyv...adhesives.html,
2006.

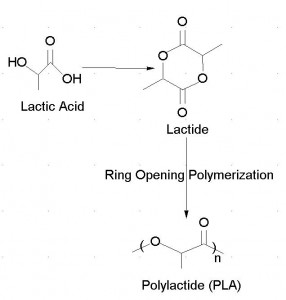
Polylactic acid (PLA) is a biodegradable plastic currently in use. Environmentalists like PLA because it is biodegradable and also made from waste products. Billions of pounds of potato peelings are wasted each year from french fries alone. Also, cheese manufactures toss away several billion pounds of cheese whey containing useable carbohydrates. Bacteria convert the carbohydrate rich waste into lactic acid. The lactic acid is then converted into PLA through condensation reactions. Another source of carbohydrates is corn, wheat and beets.
Polylactic acid is produced by poly-condensation of naturally produced lactic acid or by the catalytic ring opening of the lactide group.
Polylactic acid condensation reaction
HO-(CH(CH3)COO)n – H + HO(CH(CH3)COO)m-H → HO(CH(CH3)COOn+m + H2O
All plastics are polymers. Starch is made up of repeating glucose units held together by an α-(1→4) glycosidic bond. Starch can be formed in a condensation reaction in which water is removed when the monomers are added together. Enzymes present in plants and animals are responsible for condensation reactions. A picture of starch is shown below.

How are plastics biodegraded? They are primarily degraded by bacteria. The long chains created in the polymerization are difficult for many bacteria to degrade. They can only attack the molecule at the ends. When starch is combined with the PLA, it can be degraded more easily by the bacteria. Plasticisers such as glycerol, sorbitol and triethyl citrate are also added to starch-PLA compounds to prevent brittleness. “PLA is fully biodegradable when composted in a large-scale operation with temperatures of 60 ºC and above. The first stage of degradation of PLA (two weeks) is via hydrolysis to water-soluble compounds and lactic acid” (Shah et al., 2008). Microorganisms then break down the lactic acid into CO2, biomass and water.
From ChemPRIME: 8.24: Condensation Polymers
References:
Shah, A.; Hasan, F.; Hameed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246-265.
Sivan, A. New Perspectives in Plastic Biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422-426.
Tokiwa Y.; Clabia BP. Degradation of microbial polyesters. Biotechnol Lett. 2004, 26, 1181-9.
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


