RuBisCo
- Page ID
- 2512
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)D-Ribulose-1,5-Biphosphate Carboxylase-Oxygenase, also known as RuBisCo, is a crucial enzyme in photosynthesis due to it’s catalytic role in the Calvin Cycle via fixation of CO2 to D-Ribulose. This fixation is RuBisCo’s most noted achievement as it is a method of introducing inorganic carbon into the biosphere. It is also the most abundant enzyme found in plant leaves and is considered to be the most abundant protein on the planet.
Structure of Rubisco
The rubisco active site is arranged around a magnesium ion. The center magnesium ion connects to a small sugar molecule, three amino acids, lysine, and carbon dioxide molecules.1 The small sugar molecule that is attached to the magnesium ion is similar to the product that is produced from the Calvin Cycle.1 The lysine that the magnesium ion attaches to is a modified form of lysine. The lysine has a carbon dioxide molecule attached to its end.1 In plants, this carbon dioxide molecule is an activator that is attached to rubisco. During the daytime when there is sunlight present, the enzyme is turned on and at night when there is no light present, the enzyme is turned off. When the enzyme is turned on, the magnesium binds to ribulose bisphosphate by attaching to two oxygen atoms and the carbon dioxide molecule that is connected to the sugar.1

Figure 1 - Rubisco's large and complex form2
The form of rubisco in plants and algae is a very large and complex form as shown in Figure 1. The enzyme is made out of eight copies of a large protein chain shown in gray and white parts in Figure 1.1 There are eight copies of a smaller chain as shown in the blue and orange colored parts.1 Many enzymes have similar symmetrical complexes like rubisco but rubisco differs because it is “rigid as a rock, with each of the active sites acting independently of one another.”1
Symmetry of Rubisco

Figure 2 - A magnesium ion (shown in green) is surrounded by a sugar molecule and a lysine molecule (Hand drawn picture)
In Figure 2, the magnesium ion is surrounded by the sugar molecule along with the modified lysine. As shown in the picture, the magnesium ion is connected to six oxygen molecules. Since this center metal is attached to six molecules that are the same, the geometry of the metal is an octahedral.

Figure 3 - C4 and C2 axes and σh plane are shown on the rubisco structure (Hand drawn picture)
The immediate ligand environment for the magnesium ion in rubisco is six oxygen molecules. The point group of the metal is D4h. The molecule is not linear. Even though the geometry is octahedral, the molecule does not have an Oh symmetry because there are four long bonds and two short bonds for the six bonds present for the magnesium. The principal axis for rubisco is C4, as shown in Figure 3. The C4 goes from one of the oxygen atoms from the sugar molecule to the oxygen from the lysine. There is a C2 axis that is perpendicular to the C4 axis. Figure 3 also shows the C2 axis. The C2 axis is between four of the oxygen atoms. Two of the oxygen atoms are from the sugar molecule and the other two are from the lysine. There is a σh plane which is perpendicular to the C4 axis. This horizontal plane is also shown in Figure 3. Thus, the point group of the magnesium metal in rubisco is D4h.
The overall point group of rubisco is C1 because it has no symmetry. The molecule of rubisco is not linear and it does not have any tetrahedral, octahedral, or icosahedrons symmetry. There is no principal Cn axis and there is no mirror plane. There is also no center of inversion, which makes the point group of rubisco C1.
General Information on Rubisco
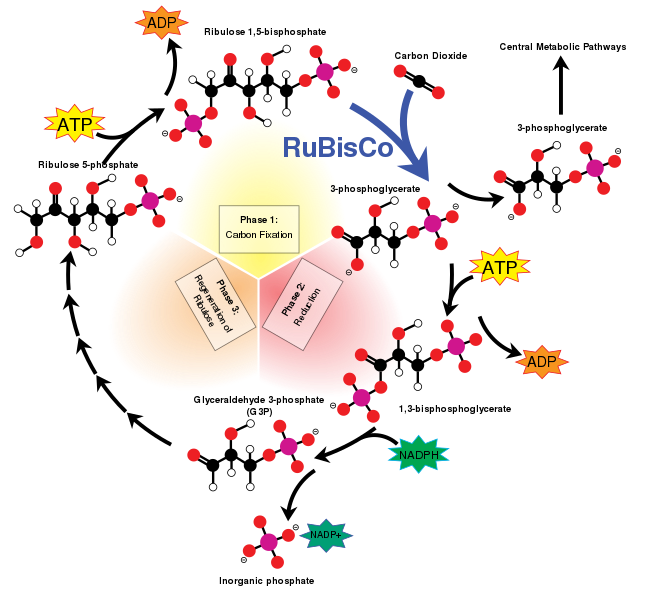
Figure 4 - The Calvin Cycle2
Rubisco is a common protein found in leaves which shows the importance rubisco has for the process of photosynthesis.3 The first step in photosynthesis is known as the Calvin Cycle which is shown in Figure 4. The Calvin Cycle, which is a “metabolic pathway found in the stroma of the chloroplast” of a plant,4 involves carbon entering the pathway in the form of carbon dioxide and leaving the pathway in the form of sugar. The rubisco enzyme’s job is to catalyze the first step of the Calvin Cycle by “creating carbon from the inorganic carbon dioxide in the air.”1 During the first step, carbon fixation occurs with the help of rubisco. The enzyme helps the carbon dioxide attach to the ribulose bisphosphate, a five-carbon sugar chain.4 The product that is produced is a six-carbon molecule that is split in half by the rubisco “to form two molecules of 3-phosphoglycerate” with three carbon atoms.4 The majority of the phosphoglycerate that is formed by rubisco is used to create more ribulose bisphosphate while some of the phosphoglycerate continues on the journey through the Calvin Cycle to make sucrose or starch for the plant.1 The Calvin Cycle continues after the help of rubisco during the first crucial step.
It is interesting to note that even though enzymes are commonly used to speed up reactions, rubisco is quite slow compared to other enzymes.4 Rubisco “can fix only a few carbon dioxide molecules per second” while other enzymes “can catalyze thousands of chemical reactions per second.”4 Plant cells are able to handle such a slow-rate enzyme inside of them by producing many rubisco enzymes,1 which explains why rubisco is so abundant in plants.
Three scientists Urs Feller, Steven J. Crafts-Brandner, and Michael E. Salvucci conducted an experiment to see what temperature the rubisco enzyme can be inhibited from catalyzing the ribulose bisphosphate reaction during the Calvin Cycle. They discovered that moderately high temperatures can inhibit rubisco. The scientists used cotton and wheat leaf tissue for their experiment. They exposed the leaves to increasing temperatures in the light and saw that the “activation of rubisco was inhibited above 35 and 30°C.”5 The inhibition for wheat was greater than cotton. The inhibition of rubisco was completely reversible at temperatures below 40°C. It is interesting how rubisco was not affected by very high temperatures such as 45°C, but it is affected by moderately high temperatures. Once rubisco is inhibited the Calvin Cycle is also inhibited. The scientists' evidence “indicates that moderately elevated temperatures inhibit light activation of rubisco.”5
References
- Goodsell, David S. “Rubisco: November 2000 Molecule of the Month.” RCSB Protein Data
Bank. November 2000. 22 May 2010. <http://www.rcsb.org/pdb/static.do?p=...h/pdb11_1.html>. - Wikipedia contributors. “Rubisco.” Wikipedia, The Free Encyclopedia. 12 Dec. 2007. Web. 23 May 2010. <http://en.wikipedia.org/wiki/RuBisCO>.
- “Rubisco.” ISCID Encyclopedia of Science and Philosophy. 2010. 22 May 2010. <http://www.iscid.org/encyclopedia/cite/Rubisco>.
- “The Calvin Cycle.” The Connecticut River. 22 May 2010. <http://www.bio.umass.edu/biology/con...er/calvin.html>.
- Feller, Urs, Steven J. Crafts-Brandner, Michael E. Salvucci. “Moderately High Temperatures
Inhibit Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) Activase-Mediated Activation of Rubisco.” Plant Physiology. 1998. 22 May 2010. <http://www.plantphysiol.org/cgi/cont...ract/116/2/539>.
Contributors
- Jenny Trinh

