14.P: Problems for Chapter 14
- Page ID
- 991
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Link to Solution Manual
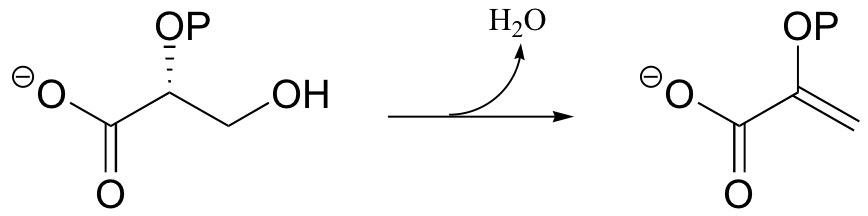
P14.1: Enolase catalyzes the reaction shown below. An active site lysine is thought to participate as a catalytic base, and a glutamic acid residue is though to participate as a catalytic acid. The enzyme requires magnesium ion to function. Propose a mechanism, showing the predicted roles of the Lys, Glu, and magnesium.
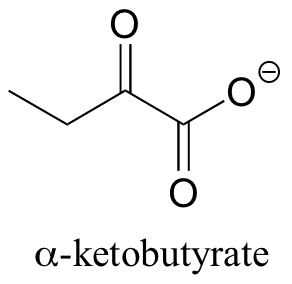
P14.2: In one of its degradation pathways, threonine is first converted to alpha-ketobutyrate through an enamine intermediate. Show a complete mechanism for this reaction.
P14.3: The amino acid aspartate undergoes elimination of ammonia as part of its degradation pathway. Show the expected result of this elimination.
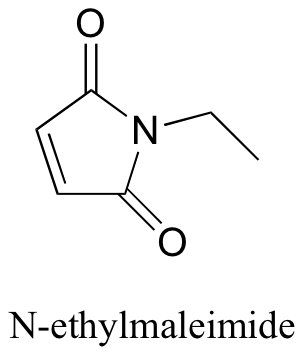
P14.4: N-ethylmaleimide is an irreversible inactivator of many enzymes that contain active site cysteines. Show how this inactivation could occur, and show the structure of the labeled cysteine.
P14.5:
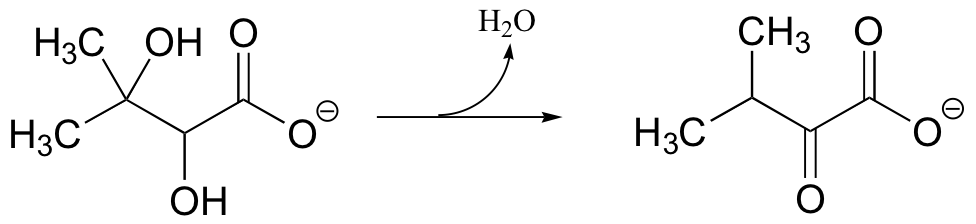
a) Provide a likely mechanism for the following reaction from the biosynthesis of the amino acid valine:
b) In a newly discovered pathway for the biosynthesis (in bacteria) of the building block compound for isoprenoid biosynthesis, pyruvate and glyceraldehyde-3-phosphate condense, with the release of carbon dioxide. The reaction requires a coenzyme. Draw a complete mechanism for this reaction, showing the product formed and the role of the coenzyme.
P14.6: Show the product and provide a mechanism for the following Michael addition reaction.
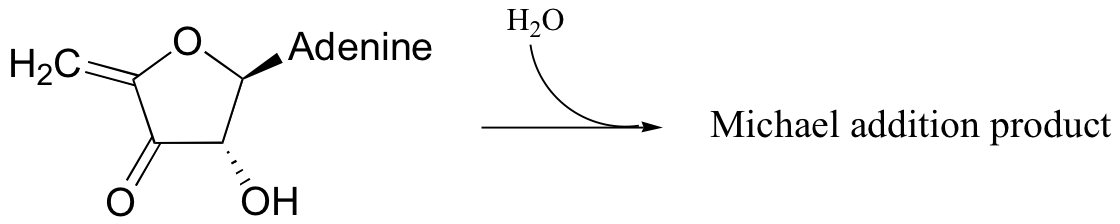
P14.7: The following substrate undergoes an E1cb dehydration followed by re-hydration to form a different constitutional isomer. Predict the structure of the isomer, and also the structure of the intermediate structure.

P14.8: Both reactions below are thought to involve elimination by the E1 mechanism.
a) Predict the product, and propose a mechanism:
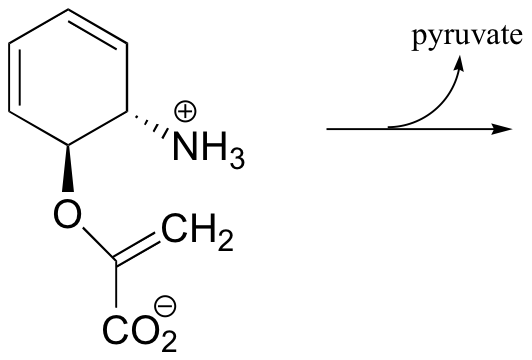
b) Propose a mechanism:
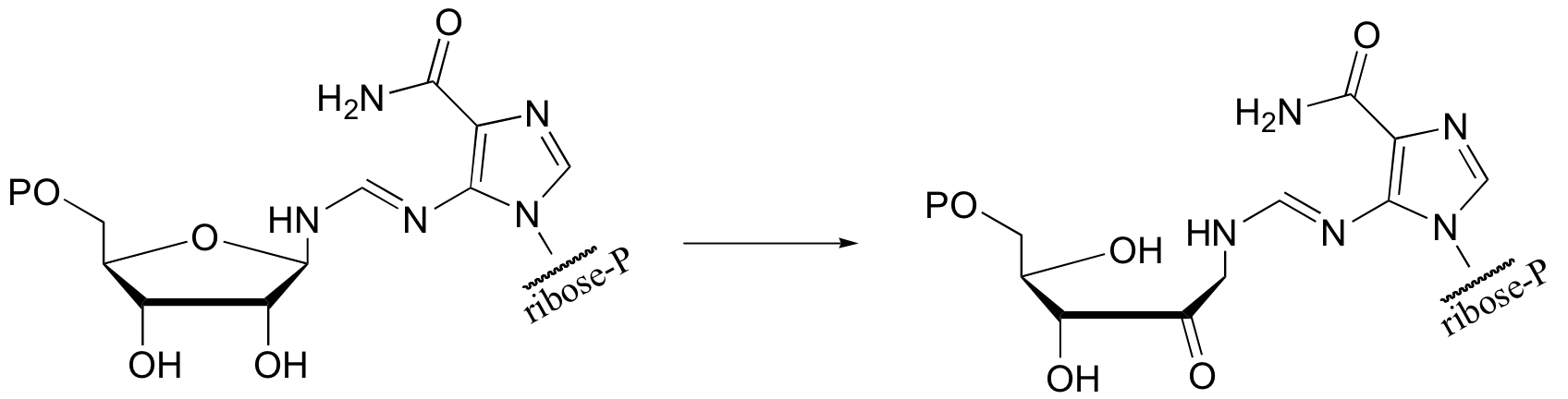
P14.9: Predict the second product for this reaction. What type of mechanism is it?
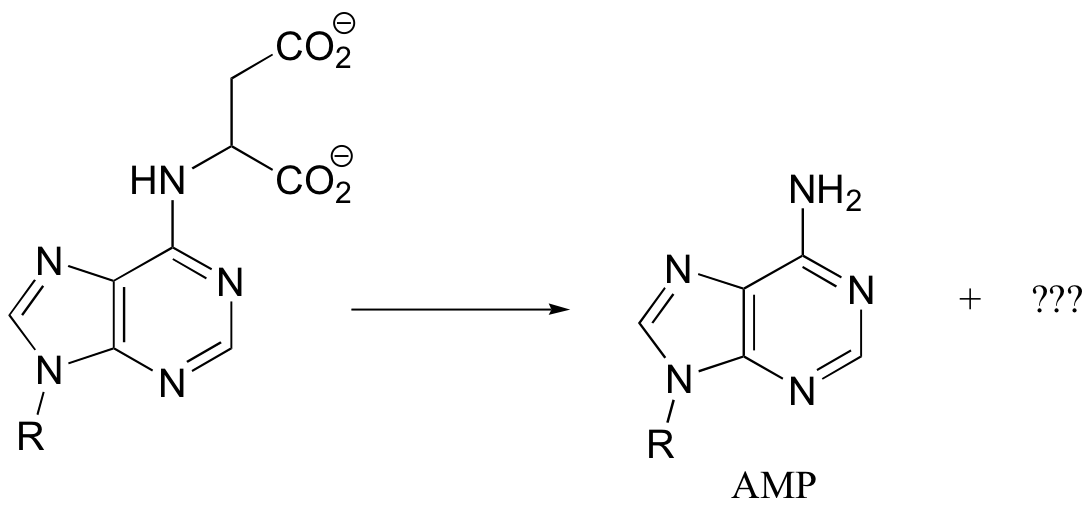
P14.10: All of the following reactions are PLP-dependent. Draw mechanisms, showing the ‘electron sink’ action of PLP. In each case, begin the substrate-PLP adduct, and end with the product-PLP adduct (in other words, you do not need to show the Schiff base being formed and later hydrolyzed)
a) threonine → glycine + acetaldehyde (IUPAC name ethanal)
b)
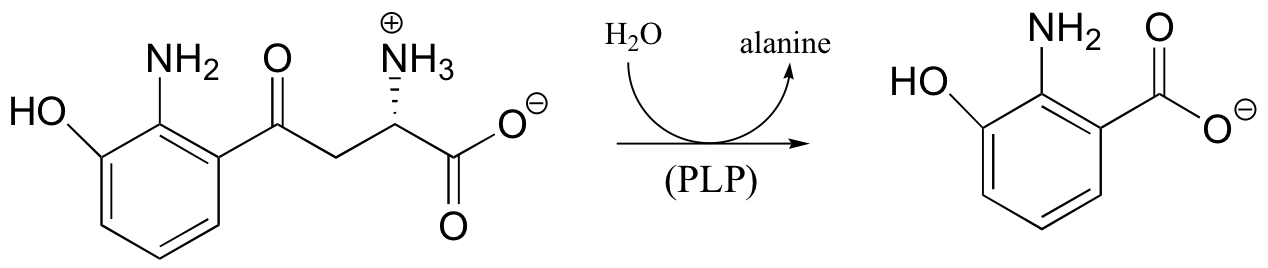
c)
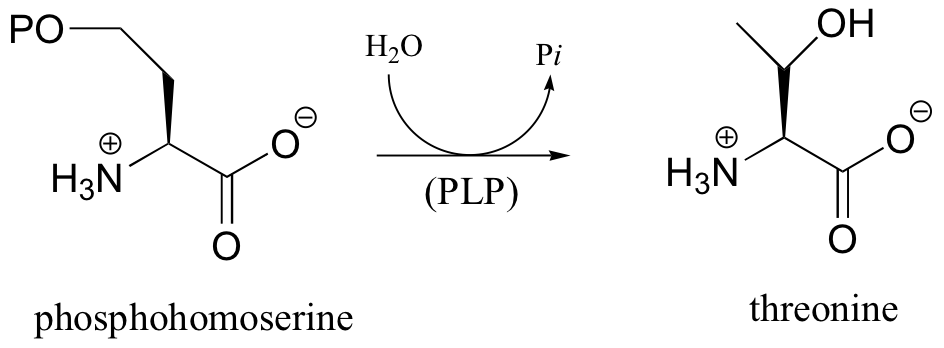
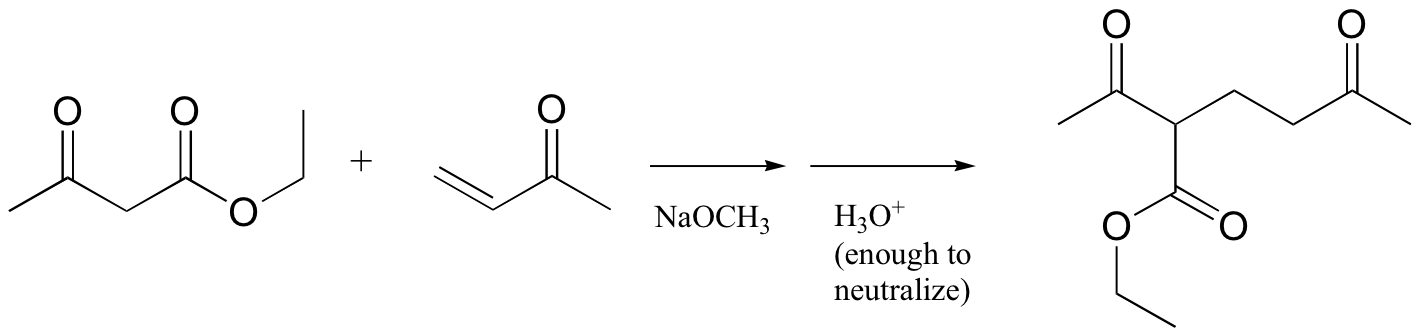
P14.11: Propose a mechanism for this laboratory reaction:
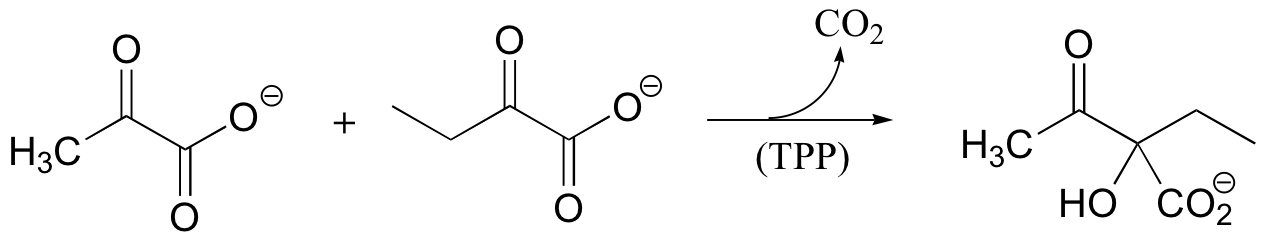
P14.12: The following reaction requires thiamine diphosphate as a cofactor. Propose a mechanism that takes into account the role of TPP as an electron sink.
P14.13: Draw all possible elimination products for the following starting materials:
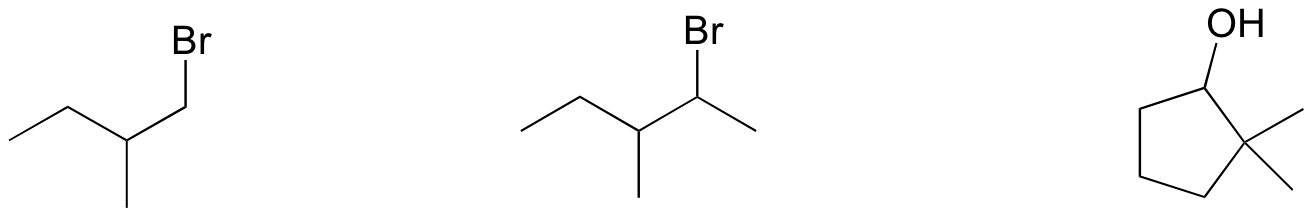
P14.14: Draw the transition state for an E2 reaction in which 2-bromobutane is converted to (E)-2-butene. Be sure to accurately show the geometry of the breaking bonds.
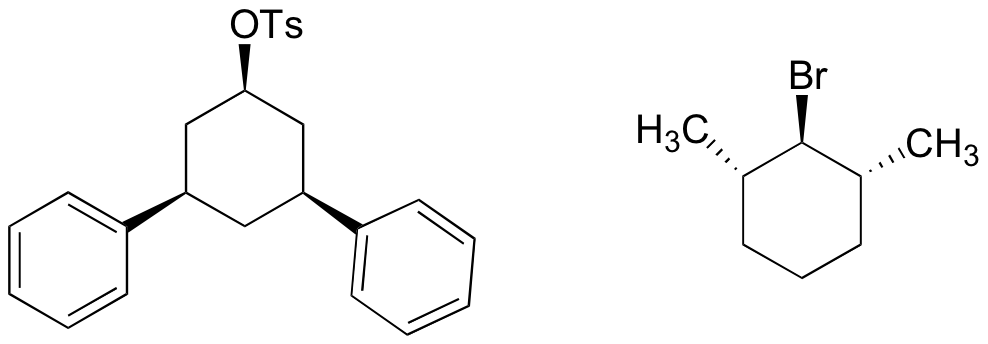
P14.15: The compounds below will not react by the E2 mechanism. Explain why not.
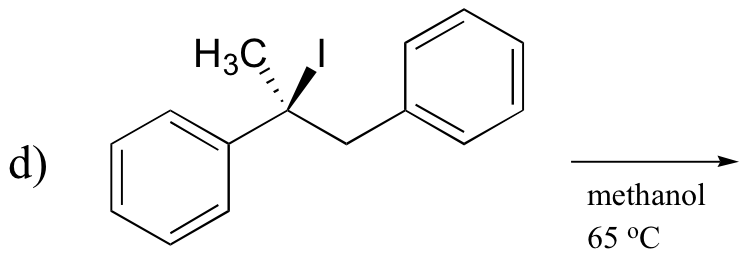
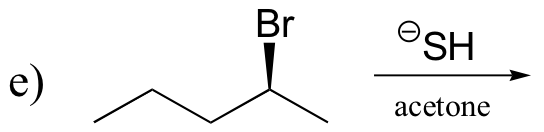
P14.16: Predict the most abundant product of each of the following (nonenzymatic) reactions, and state the mechanism by which the product forms.
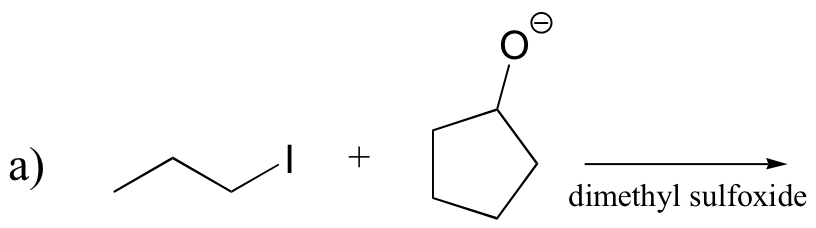

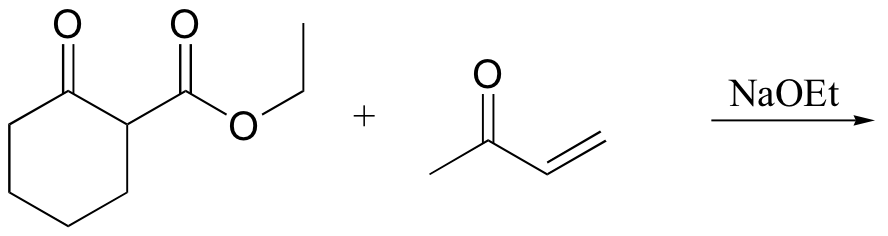
P14.17: Draw a complete mechanism for the Robinson annulation reaction discussed in section 14.1D.
P14.18: Predict the products of the following Robinson annulation reactions:
a)
b)
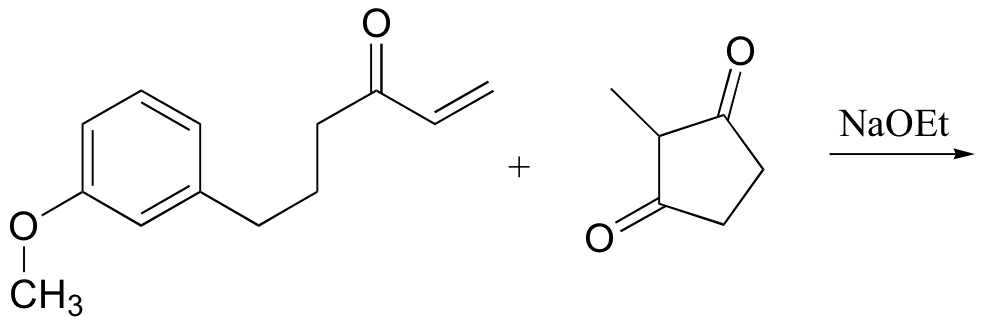
P14.19: Show the starting materials that you could use to synthesize the following compounds by Robinson annulation:
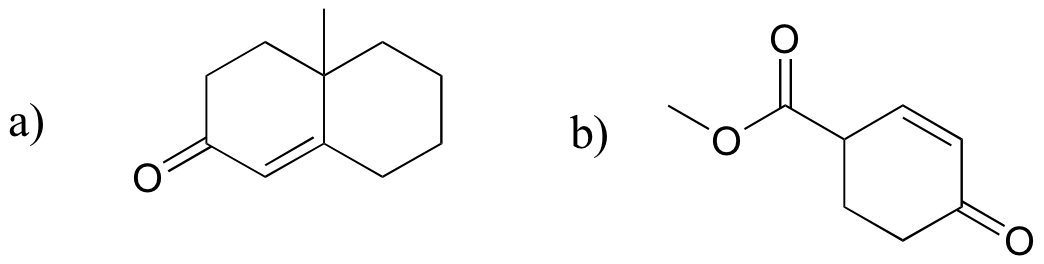
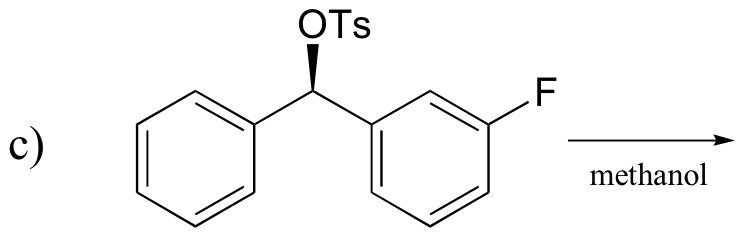
P14.20: Predict the product of these laboratory reactions:
a)
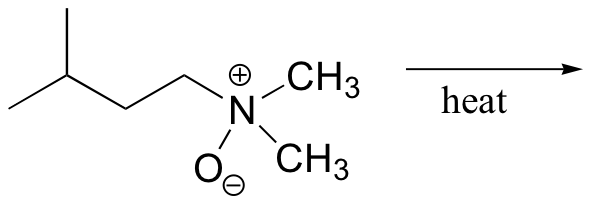
b)
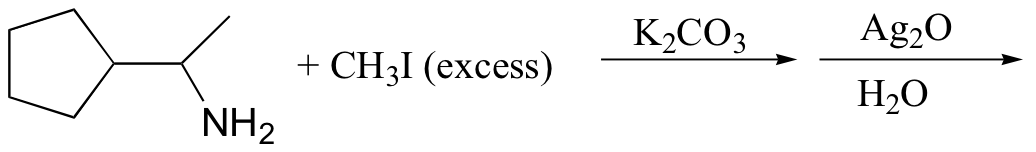
c)
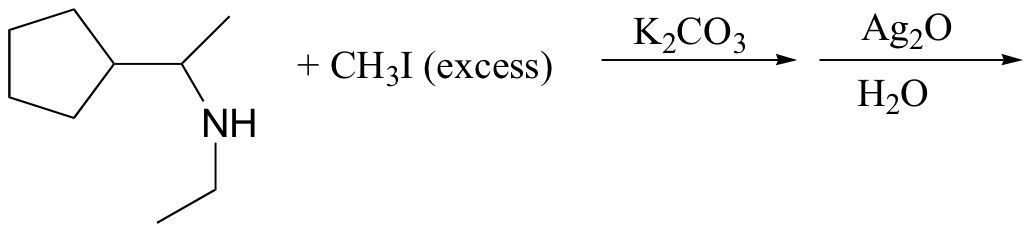
Challenge problems
C14.1: The reaction shown below, catalyzed by orotidine monophosphate decarboxylase, is one of the most extensively studied enzymatic transformations. It is known to occur without the participation of any coenzymes.
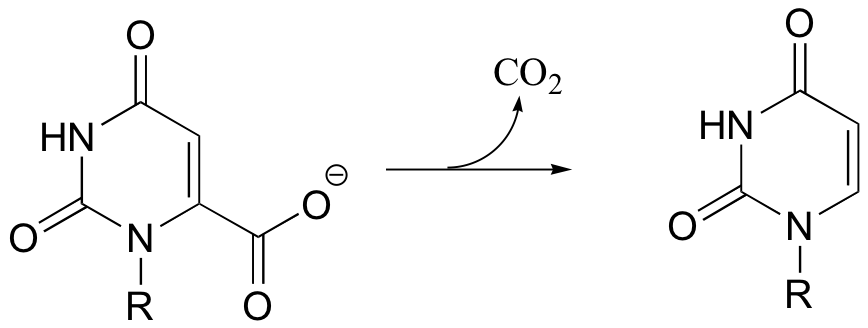
a) Look at the reaction closely: what is unique about it?
b) In 1997, a paper was published in which the authors predicted, based on theoretical calculations, that this reaction proceeded through a carbene intermediate (carbenes are not covered in this text – you may need to look them up). This was prior to the publication of an x-ray crystal structure. What kind of active site environment does this imply?
c) When the crystal structure was published a few years later, we learned that an aspartate residue (predicted to be negatively charged) is positioned very near the substrate carboxylate group, and a lysine residue (predicted to be positively charged) is positioned nearby on the opposite side (see figure below). What roles do you think were predicted for these two active site residues?
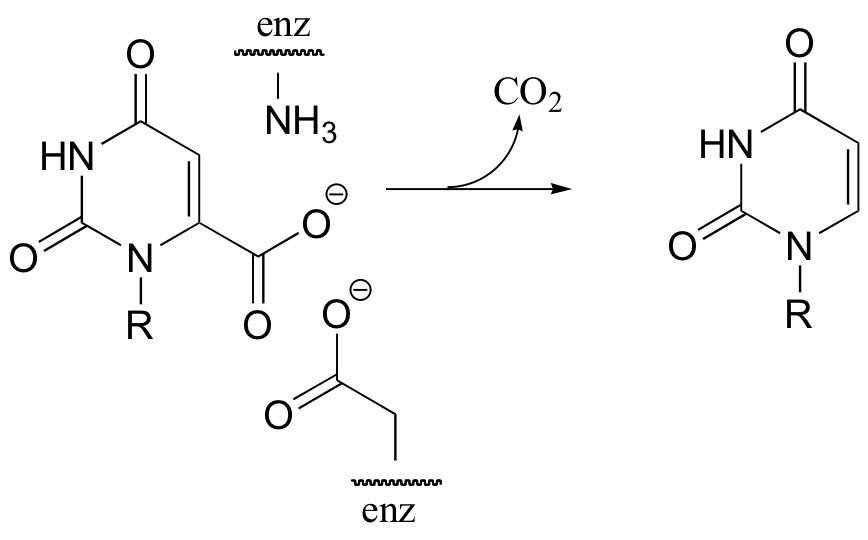
C14.2: 5,10-methylene tetrahydrofolate is a coenzyme that, like S-adenosylmethionine (SAM), serves to transfer single carbons. Show a likely mechanism for the transformation shown below.
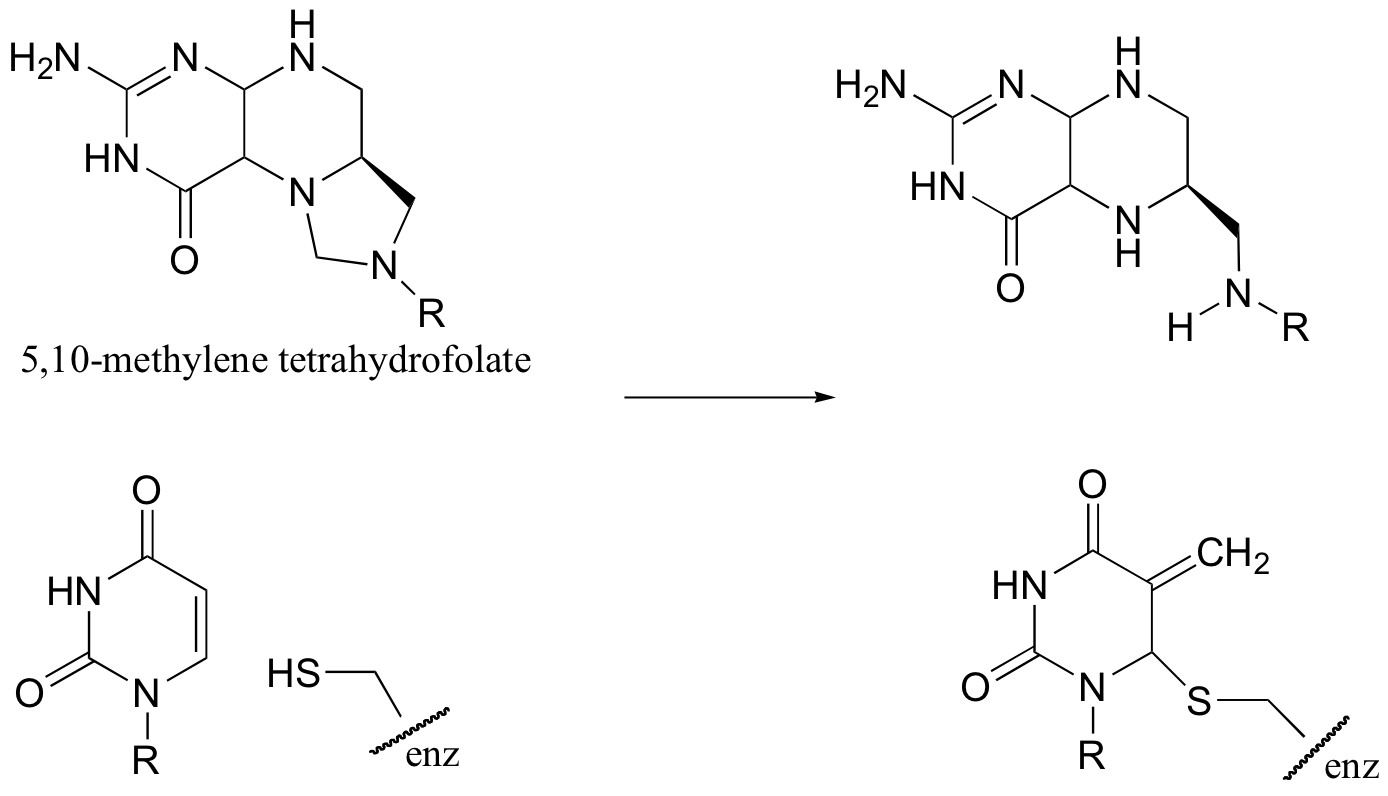
C14.3: Suggest a mechanism for the following reaction:
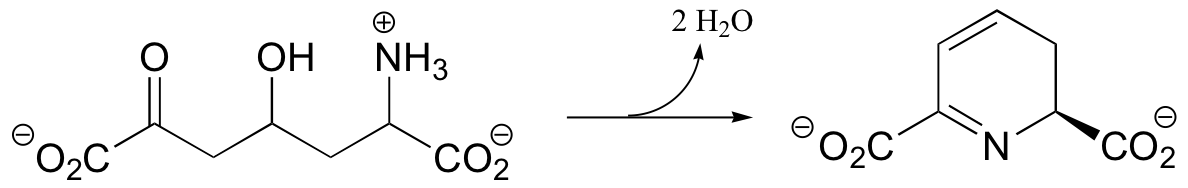
C14.4:
a) Given the transformation shown below (and no other information), predict a likely mechanism based on similar transformations we have seen previously.
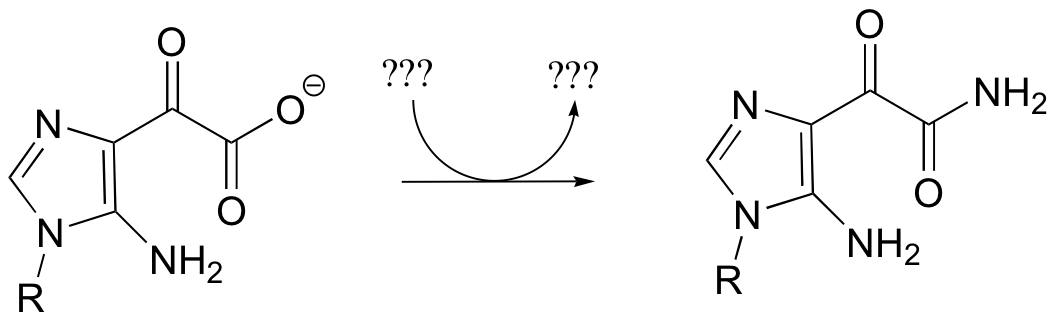
b) As is turns out, this reaction requires ATP and aspartate, and releases ADP, Pi, and fumarate. Given this information, predict a different mechanism.
<style type="text/css"></style>
C14.5: In the histidine degradation pathway, histidine undergoes elimination of ammonia to form trans-urocanate in what, at first glance, appears to be an E1-like mechanism similar to the dehydration reaction from histidine biosynthesis, detailed in section 14.3B. However, the enzyme catalyzing this reaction has been shown to use an unusual 'coenzyme', 4-methylideneimidazole-5-one (MIO), which is formed from the cyclization of an alanine-serine-glycine stretch of the enzyme itself. A mechanism has been proposed in which the MIO coenzyme plays the role of electron sink, and the intermediate shown below forms.
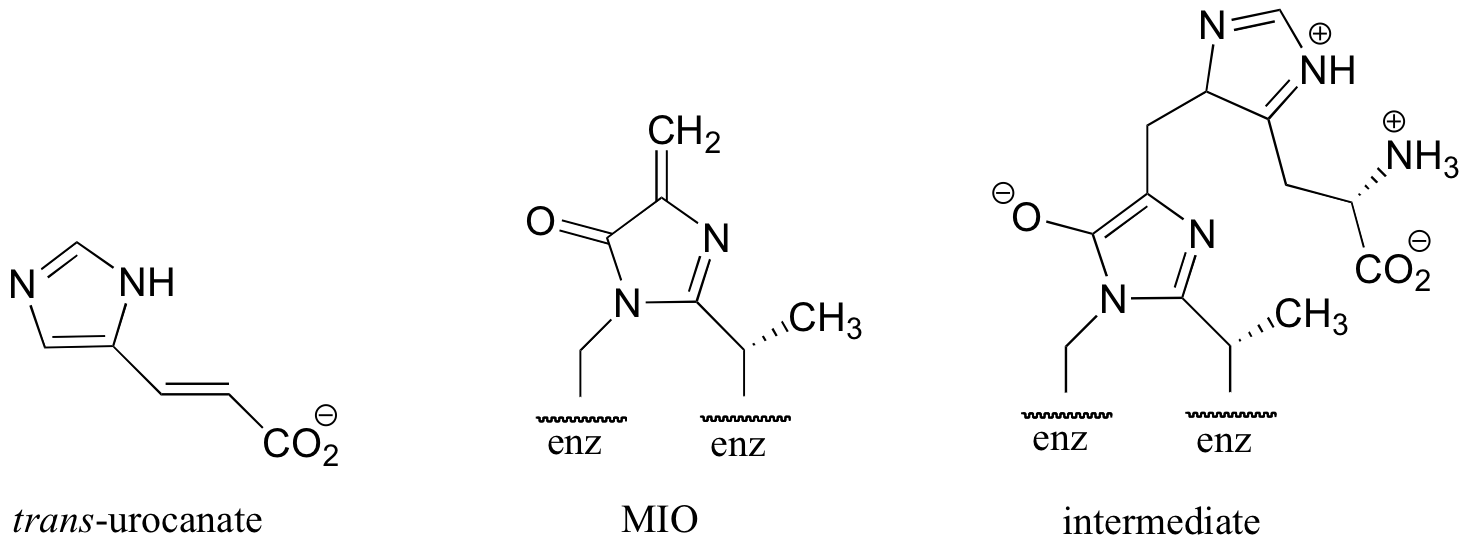
Propose a full mechanism for this reaction according to this information.


