Extra Credit 5
- Page ID
- 83284
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.5
Given the following pairs of balanced half-reactions, determine the balanced reaction for each pair of half-reactions in an acidic solution.
- Ca ⟶ Ca2++2e−, F2+2e−⟶ 2F−
- Li ⟶ Li++e−,Cl2+2e−⟶ 2Cl−
- Fe ⟶ Fe3++3e−,Br2+2e−⟶ 2Br−
- Ag⟶Ag++e−, MnO−4+4H++3e−⟶ MnO2+2H2O
S17.1.5
a)
1) Because both half reactions are balanced and contain the same number of electrons on each side when added together, the balanced reaction is:
Ca ⟶ Ca2++2e−, F2+2e−⟶ 2F−
2) Since both sides have 2 electrons, we can cancel them out and get a final answer:
Ca + F2 ⟶ Ca2++2F−
The Ca ⟶ Ca2++2e− reaction is oxidation as calcium goes from being neutral to having a +2 charge.
The F2+2e−⟶ 2F− reaction is reduction as fluorine goes from being neutral to having a -1 charge.
b)
1) Split each half reaction into reduction half and oxidation half:
reduction (because Cl is gaining electrons):
Cl2+2e−⟶ 2Cl−
oxidation (because Li is losing electrons):
Li ⟶ Li++e−
2) Because the number of electrons is not the same for both half reactions we must multiply the oxidation half-reaction by a factor of 2, because the number of electrons lost by one species must be equal to the number gained by the other species:
2(Li ⟶ Li++e−)
3) Now, because we have the same number of electrons in each half reaction, we can add the equations together:
2Li + Cl2+ +2e- ⟶ 2Li+ + 2Cl− + 2e-
4) Canceling out the electrons gives us:
2Li + Cl2 ⟶ 2Li+ + 2Cl−
c)
1) Split each half reaction into reduction half and oxidation half:
reduction (because Br is gaining electrons):
Br2+2e−⟶ 2Br−
oxidation (because Fe is losing electrons):
Fe ⟶ Fe3++3e−
2) Because the number of electrons is not the same for both half reactions we must multiply the oxidation half-reaction by a factor of 2 and the reduction half-reaction by a factor of 3 so each equation can have 6 electrons- the number of electrons lost by one species must be equal to the number gained by the other species:
3(Br2+2e−⟶ 2Br−)
2(Fe ⟶ Fe3++3e−)
3) Now, because we have the same number of electrons in each half reaction, we can add the equations together and the electrons cancel out:
3Br2+2Fe ⟶ 2Fe3++6Br−
d)
1) Split each half reaction into reduction half and oxidation half:
reduction (gains electrons):
MnO−4+4H++3e−⟶ MnO2+2H2O
oxidation(loses electrons):
Ag ⟶ Ag++e−
2) Multiply oxidation half-reaction by a factor of 3 to get the same number of electrons on each side:
3(Ag ⟶ Ag++e−)
3) Add equations together and cancel electrons:
MnO4+4H++3Ag⟶3Ag++MnO2+2H2O
Q19.1.3
Write the electron configurations for each of the following elements and its 3+ ions:
- La
- Sm
- Lu
S19.1.3
a)
1) After locating La on the periodic table, start by writing the noble gas (Xenon) that comes before La on the table in square brackets:
[Xe]
2) Then we write 6s2 because La has two electrons in the 6s orbital. (6s because n= 6)
[Xe] 6s2
3) Then we write 5d1 because it has one electron in the 5d orbital. (5d because n-1 = 5)
[Xe] 6s2 5d1
For La3+:
use the same configuration from part 1, but since it loses 3 electrons, we take off the two electrons from the s orbital and the one electron from the d orbital. We are then left with the noble gas configuration that is represented just as [Xe].
- When writing the electron configuration for cations, always remove electrons from the s orbital before the d orbital because the d orbital is higher in energy.
[Xe]
b)
1) Locate Sm on the periodic table and write the noble gas that comes before it on the periodic table: Xenon.
[Xe]
2) Write 6s2 because there are two electrons in the 6th subshell of the s orbital.
[Xe] 6s2
3) Then we add 4f6 because there are 6 electrons in the f orbital (4f because n-2 = 4).
[Xe] 6s2 4f6
For Sm3+:
1) Write the same thing as part 1:
[Xe] 6s2 4f6
2) Rewrite the configuration in increasing n:
[Xe] 4f6 6s2
3) Take 3 electrons off starting with the electrons in the 6s orbital first, and then take off one electron from the 4f orbital:
[Xe] 4f5
c)
1) Locate Lu on the periodic table and write the noble gas that comes before it on the periodic table: Xenon.
[Xe]
2) Write 6s2 because there are two electrons in the 6th subshell of the s orbital:
[Xe] 6s2
3) Then we add 5d1 because there is 1 electron in the d orbital (5d because n-1 = 5).
[Xe] 6s2 5d1
4) Then write 4f14 because there are 14 electrons in the f orbital (4f because n-2 = 4).
[Xe] 6s2 5d1 4f14
For Lu3+:
Write what we wrote for part 1. Then we write configuration by increasing n. Then take off 3 electrons from right to left. That leaves us with:
[Xe] 4f14
Q19.2.5
Draw diagrams for any cis, trans, and optical isomers that could exist for the following (en is ethylenediamine):
- [Co(en)2(NO2)Cl]+
- [Co(en)2Cl2]+
- [Pt(NH3)2Cl4]
- [Cr(en)3]3+
- [Pt(NH3)2Cl2]
S19.2.5
1. cis- This isomer is cis because both (en) groups are located next to each other. They are on different axes in three-dimensional space.
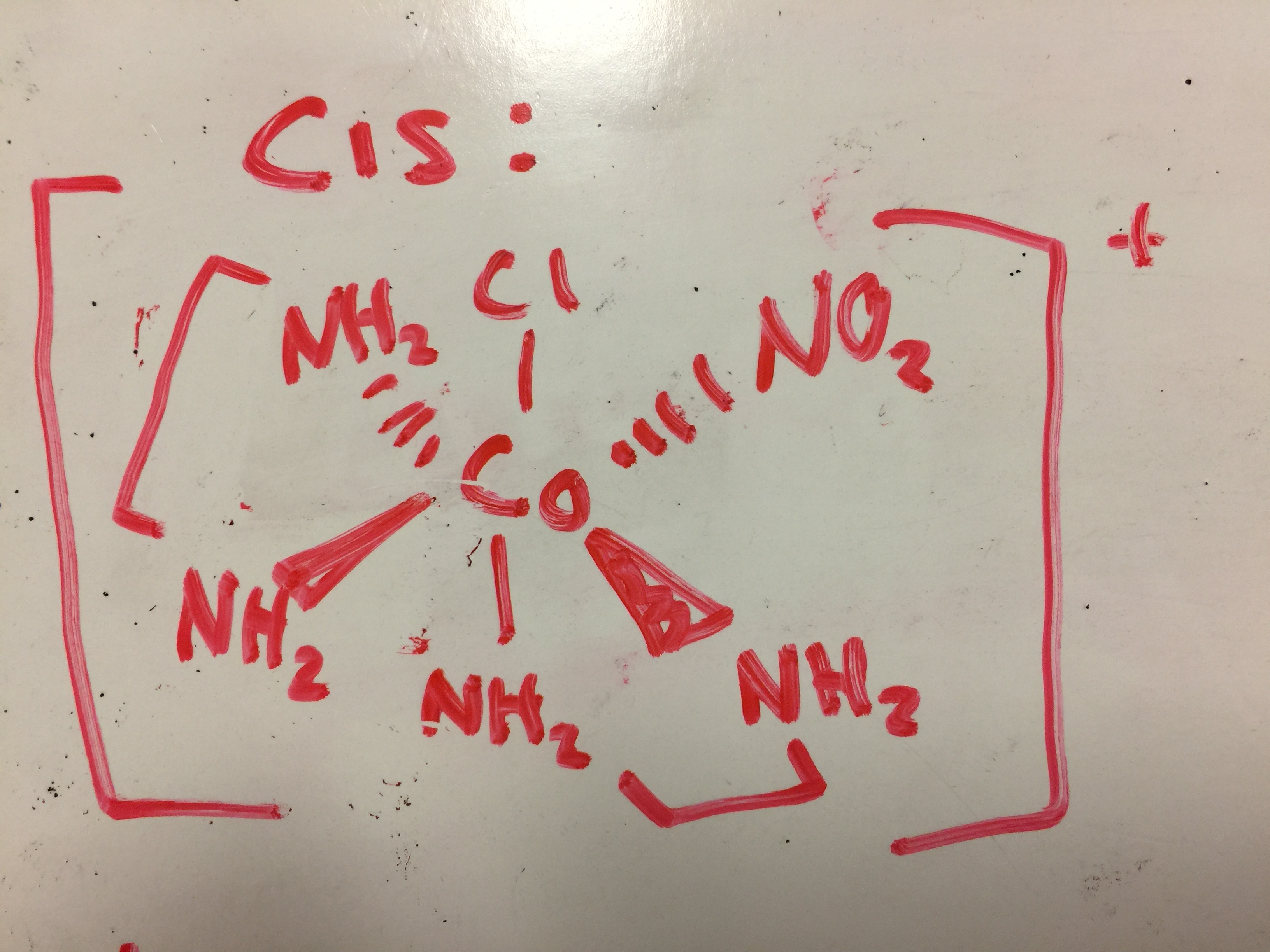
2. trans- This isomer is trans because the (en) groups are on opposite sides of the central ion. They are on the same axis in three-dimensional space.

2.
1. This complex has one trans isomer because (en) lies on the same axes:
2. This complex has two cis isomers. They are both chiral because they are both non-superimposable. They are both cis because (en) lies on different axes:


3. cis and trans isomers possible.
4. No isomers because it has a line of symmetry and all ligands are the same.
5.
1. The complex is square planar so it has no optical isomers.
2. Trans because the ligands are on the same axes:
3. Cis because the ligands are on opposite axes:
Q12.3.18
For the reaction A⟶B+CA⟶B+C, the following data were obtained at 30 °C:
| [A] (M) | 0.230 | 0.356 | 0.557 |
|---|---|---|---|
| Rate (mol/L/s) | 4.17 × 10−4 | 9.99 × 10−4 | 2.44 × 10−3 |
- What is the order of the reaction with respect to [A], and what is the rate equation?
- What is the rate constant?
a)
1) Choose 2 points from data set:
(.230, 4.17E-4), (.557, 2.44E-3)
2) Set up ratio of concentrations with an unknown exponent equal to ratio of corresponding rates:$$(\frac{0.557}{0.230})^x=(\frac{2.44\times10^{-3}}{4.17\times10^{-4}})$$
3) Take log of both sides and solve for x which is equal to the order of the reaction with respect to A. $$x=\frac{\log(\frac{2.44\times10^{-3}}{4.17\times10^{-4}})}{\log\frac{0.557}{0.230}}$$
x = 2
b) Since we know that the order of the reaction with respect to A is second order, the corresponding rate law equation is: r = k [A]2.
We can then pick any of the three provided trials from the data to solve for k by plugging in the rate and concentration of A:$$4.17\times10^{-4}=k\times[.23]^2$$
For Trial 1 data used here, k = 7.88E-3
Q12.6.9
Consider the reaction
CH4 + Cl2 → CH3Cl + HCl (occurs under light)
The mechanism is a chain reaction involving Cl atoms and CH3 radicals. Which of the following steps does not terminate this chain reaction?
- CH3 + Cl → CH3CI
- CH3 + HCl → CH4 + Cl
- CH3 + CH3 → C2H2
- Cl + Cl → Cl2
S12.6.9
Number 3 because C2H2 is a potential intermediate and is not listed in the original equation.
Q21.4.21
A sample of rock was found to contain 8.23 mg of rubidium-87 and 0.47 mg of strontium-87.
- Calculate the age of the rock if the half-life of the decay of rubidium by β emission is 4.7 × 1010 y.
- If some$$Sr_{38}^{37}$$was initially present in the rock, would the rock be younger, older, or the same age as the age calculated in (a)? Explain your answer.
a)
1) solve for decay constant:$$\lambda=\frac{ln(2)}{t_{1/2}}\rightarrow\frac{ln(2)}{4.7\times10^{10}}=1.47\times10^{-11}yr^{-1}$$
2) One mole of rubidium decomposed per mole of strontium produced.
The initial number of moles of rubidium equals number of moles of radium left added to the number of moles of strontium: $$\frac{8.23\times10^{-3}g}{87 g/mol}+\frac{.47\times10^{-3} g}{87 g/mol} = 9.96\times10^{-5}mol$$
3) plug in 10-4 years for No, 9.46 x 10-5 years for Nt, and 1.47 x 10^-11 years^-1 for in the equation:$$t=(\frac{1}{\lambda})\times(\ln\frac{N_t}{N_o})$$
$$\frac{1}{1.47\times10^{-11}yrs^-1}\times\ln\frac{10\times{-4} yrs}{9.46\times10^{-5} yrs}=3.8\times10^{9} yrs$$
b) Since time is directly proportional to the amount of strontium, the rock would be younger than the actual calculated age if there was some initial amount of strontium present when the rock was formed.
Q20.3.9
The reaction Pb(s)+2VO2+(aq)+4H+(aq)→Pb2+(aq)+2V3+(aq)+2H2O(l) occurs spontaneously.
- Write the two half-reactions for this redox reaction.
- If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which reaction occurs at the cathode and which occurs at the anode?
- Which electrode is positively charged, and which is negatively charged?
S.20.3.9
a) Look at which species is losing electrons and which is gaining to separate the equations into two half reactions.
oxidation (loss of electrons): Pb(s) --> Pb2+(aq) + 2e-
reduction (gain of electrons): 2VO2+(aq) + 4H+(aq) + 2e- --> 2V3+(aq) + 2H2O(l)
b)The Pb half reaction is occurring at the anode because it is losing electrons and oxidation ALWAYS happens at the anode in a galvanic cell. The other reaction is occurring at the cathode because VO2+ is gaining electrons (reduction ALWAYS happens at the cathode in a galvanic cell).
c)The cathode is positively charged because it has to attract the electrons moving from the negatively charged anode that is repelling them.
Q20.5.20
The standard electrode potential (E°) for the half-reaction Ni2+(aq) + 2e− → Ni(s) is -0.257 V. What pH is needed for this reaction to take place in the presence of 1.00 atm H2(g) as the reductant if [Ni2+] is 1.00 M?
S.20.5.20
1. Write the reduction reaction given and the equation for the oxidation of hydrogen along with their standard E cell values. Since the E° cell for hydrogen is 0, then the standard E cell for the total reaction is -0.257.
Ni2+(aq) + 2e− → Ni(s), E° cell=-0.257 V
H2→2H++2e-, E° cell=0
The standard reduction potential for the corresponding hydrogen half-reaction can be found in an SRP table.
2. Use equation:$$E cell=E° cell-(\frac{0.0591}{2})\times\ln{k}$$to solve for the concentration of hydrogen needed. We can use this formula with K, the equilibrium constant because 1 M indicates standard conditions. Plug in the standard conditions values and set equation to greater than 0 because E°cell needs to be positive in order for the reaction to be spontaneous.
$$E cell=E° cell-(\frac{0.0591}{2})\times(\ln\frac{x}{1 M})\gt0$$
$$x\times[H^+]\lt.3679$$
3. After solving for the concentration of hydrogen, convert it into pH by taking the negative log of that value.
$$pH=-\log{.3679}$$
pH = .434
4. pH less than .434 is needed to make reaction spontaneous.



