Extra Credit 4
- Page ID
- 83273
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Q17.1.4
For each of the following unbalanced half-reactions, determine whether an oxidation or reduction is occurring.
Tutorial:
oxidation definition: loss of electrons, therefore increasing the oxidation number
reduction definition: gain of electrons, therefore decreasing the oxidation number
-the sum of the oxidation numbers in a compound is zero
-the sum of the oxidation numbers in a polyatomic ion is equal to the charge
Solutions:
1. \(Cl^−⟶Cl_2\)
\(Cl^-\) has an oxidation number of negative one, as it is equal to the charge. Both of the chlorines in \(Cl_2\) have a charge of zero. Therefore, there was an increase in the oxidation number from negative one to zero so it is oxidation.
2. \(Mn^+2⟶MnO_2\)
\(Mn^+2\) has an oxidation number of plus two, as it is equal to the charge. Since \(O\) has an oxidation number of negative two, there is a total charge of negative four from both of the oxygens. Therefore, the \(Mn\) has to have a plus four charge to balance out the negative four charge. Since the \(Mn\) increased in oxidation number from two to four, it is oxidation.
3. \(H_2⟶H^+\)
The hydrogens in the H2 have an oxidation number of zero. \(H^+\) has an oxidation number of plus one. Therefore there was an increase in oxidation number, and it is oxidation.
4. \(NO_3^-⟶NO\)
Each of the oxygens have an oxidation number of negative two. Since the charge of \(NO_3^-\) negative one, the charge of the nitrogen is plus five to balance the charges. The oxidation number of nitrogen in \(NO\) is plus two. Therefore, the oxidation number of nitrogen decrease, and it is reduction.
Answers:
(a) oxidation; (b) oxidation; (c) oxidation; (d) reduction
Q19.1.2
Write the electron configurations for each of the following elements and its ions:
Tutorial:
Electrons are distributed into molecular orbitals, the \(s, p, d, and f\) blocks. An orbital will have a number in front of it and a letter that corresponds to the block. The s block holds two electrons, the p block holds six, the d block holds ten, and the f block holds fourteen. So, based on the number of electrons an atom has, the molecular orbitals are filled up in a certain way. The order of the orbitals is \(1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p\). An exponent will be put after the letter for each orbital to signify how many electrons are in that orbital. Noble gas notation can also be used by putting the noble gas prior to the element you are writing the configuration for, and then proceed by writing the orbitals filled after the noble gas. Metal ions of the d-block will have the two electrons removed from the s block prior to any electrons being removed from the proceeding d-block.
Solutions:
1. \(Ti\)
Titanium has an atomic number of 22, meaning it has 22 electrons. The noble gas prior to Titanium is Argon. Looking at row 4 of the periodic table, Titanium still has 4 electrons to be placed in orbitals since Argon has 18 electrons that are already placed. The remaining electrons will fill the \(4s\) orbital and the remaining two electrons will go into the \(3d\) orbital. [Ar]4s23d2
2. \(Ti^+2\)
This is an ion with a plus 2 charge, meaning 2 electrons have been removed. The electrons will be removed from the \(4s\) orbital and the 2 remaining electrons will be placed in the \(3d\) orbital. Like number 1, the prior noble gas is Argon. [Ar]3d2
3. \(Ti^+3\)
This is an ion with a plus 3 charge, meaning 3 electrons have been removed. The first 2 electrons will be removed from the \(4s\) orbital, and the third will be taken from the \(3d\) orbital, and the 1 remaining electron will be placed in the 3d orbital. Like number 1, the prior noble gas is Argon. [Ar]3d1
4. \(Ti^+4\)
This is an ion with a plus 4 charge, meaning 4 electrons have been removed. The first 2 electrons will be removed from the \(4s\) orbital and the second 2 will be removed from the \(3d\) orbital. This results in the ion having the same electron configuration as Argon. [Ar]
Answers:
- \([Ar]4s^23d^2\)
- \([Ar]3d^2\)
- \([Ar]3d^1\)
- \([Ar]\)
Q19.2.4
Sketch the structures of the following complexes. Indicate any cis, trans, and optical isomers.
Tutorial:
Cis and trans are a type of geometric isomer, meaning there is a difference in the orientation in which the ligands are attached to the central metal. In cis, two of the same ligands are adjacent to one another and in trans, two of the same ligands are directly across from one another.
Optical isomers → have the ability to rotate light, optical isomers are also chiral. Only chiral complexes have optical isomers
Chiral → asymmetric, structure of its mirror image is not superimposable
Enantiomers: chiral optical isomers (compound can have multiple enantiomers)
Tetrahedral complex with 4 distinct ligands → always chiral
-
For tetrahedral, if 2 ligands are the same, then it cannot be chiral, has a plane of symmetry
Solutions:
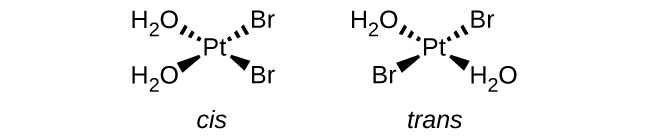
1. \([Pt(H_2O)_2Br_2]\) (square planar)
This complex has 2 kinds of ligands. The matching ligands can either be adjacent to each other and be cis, or they can be across from each other and be trans.
2. \([Pt(NH_3)(py)(Cl)(Br)]\) (square planar, py = pyridine, \(C_5H_5N\))
This complex has 4 different ligands. There is no plane of symmetry in any of the enantiomers, making the structures chiral and therefore optical isomers.
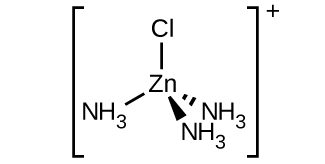
3. \([Zn(NH_3)_3Cl]^+\) (tetrahedral)
There is a plane of symmetry from \(NH_3\) through \(Zn\) to the other \(NH_3\), therefore it is not chiral.
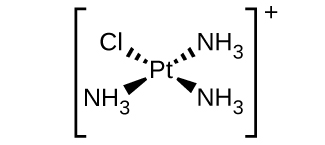
4. \([Pt(NH_3)_3Cl]^+\) (square planar)
There is a plane of symmetry from \(NH_3\) through \(Pt\) to the other \(NH_3\), therefore it is not chiral.
5. \([Ni(H_2O)_2Cl_2]\)
The \(Cl\) ligands can either be right next to each other, or directly across from one another allowing for both cis and trans geometries.
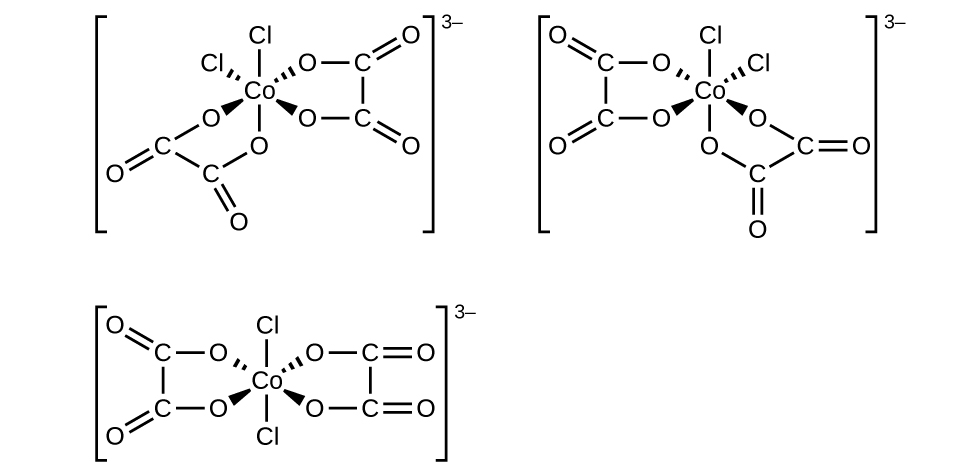
6. \([Co(C_2O_4)_2Cl_2]^-3\) (note that \(C_2O_4^-2\) is the bidentate oxalate ion, \(^−O_2CCO_2^-\)
There is a plane of symmetry from \(Cl\) through Co to the other \(Cl\), therefore it is not chiral.
Answers:
a.
b.

c.
d.
e.
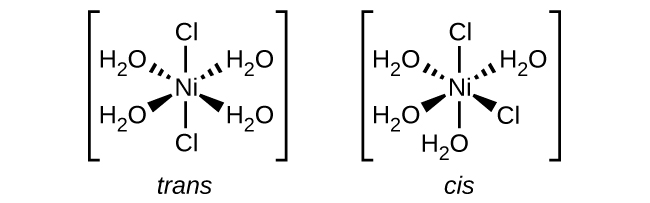
f.
Q12.3.17
Hydrogen reacts with nitrogen monoxide to form dinitrogen monoxide (laughing gas) according to the equation:
\([\ce{H2}(g)+\ce{2NO}(g)⟶\ce{N2O}(g)+\ce{H2O}(g)]\)
Determine the rate equation, the rate constant, and the orders with respect to each reactant from the following data:
| [NO] (M) | 0.30 | 0.60 | 0.60 |
|---|---|---|---|
| [H2] (M) | 0.35 | 0.35 | 0.70 |
| Rate (mol/L/s) | 2.835 × 10−3 | 1.134 × 10−2 | 2.268 × 10−2 |
Tutorial:
Steps for determining the rate law, the rate constant, and the orders.
1. The rate law shows the dependence of the rate on the concentrations of the reactants. The rate equals the rate constant times the concentrations of the reactions to the power of their order. For example, \(rate = k[A]^m[B]^n\). Write this equation for the given reaction, instead putting the reactants of the reaction in place of \(A\) and \(B\).
2. Next find the orders of all of the reactants using the data provided in the table. Add these exponents to your equation.
-
If the concentration of a compound doubles and the rate doubles → rate order is 1
-
If the concentration of a compound doubles and the rate quadruples → rate order is 2
-
If the concentration of a compound is quadrupled and the rate doubles → rate order is ½
-
If the concentration does not affect the rate → rate is zero order
3. Next find the value of k. Choose any, of the columns of the table and fill in the rate provided, concentrations provided, and and the orders as exponents you determined in step 2. Solve for k.
4. Now you can write your complete rate law.
Solution:
1. \(rate = k[NO]^?[H_2]^?\)
2. Looking at the first 2 columns for \(NO\), where \(NO\) doubles and the concentration of \(H_2\) stays the same, the rate quadruples. Therefore, the order for \(NO\) is 2. Looking at the columns 2 and 3 for \(H_2\), where \(H_2\) doubles and the concentration of \(NO\) stays the same, the rate doubles. Therefore, the order for \(H_2\) is 1. \(rate = k[NO]^2[H_2]^1
3. Using information from column 1, \(2.835×10^−3= k[0.30]^2[0.35]\) .
\(k = 0.09\)
4. \(rate = 0.09[NO]^2[H_2]^1\)
Answers:
order for \(NO\) is 2
order for \(H_2\) is 1
\(k = 0.09\)
\(rate = 0.09[NO]^2[H_2]^1\)
Q12.6.8
Nitrogen(II) oxide, \(NO\), reacts with hydrogen, \(H_2\), according to the following equation:
Tutorial:
The reaction mechanism describes the transformation, steps, and bonds broken/formed to get to the final products. A reaction mechanism has a corresponding rate law. The rate law is determined by the slowest step in the reaction mechanism, also called "the rate determining step". The rate law shows the dependence of the rate on the concentrations of the reactants. The rate equals the rate constant times the concentrations of the reactions to the power of their order. For example, \(rate = k[A]^m[B]^n\). Write this equation for the given reaction, instead putting the reactants of the reaction in place of A and B. The rate law can only include reactants and products from the final balanced chemical equation. If any intermediates are a part of the rate determining step, they must be substituted for products and reactants in the final balanced chemical equation.
\(2NO+2H_2⟶N_2+2H_2O\)
What would the rate law be if the mechanism for this reaction were:
\(2NO+H_2⟶N_2+H_2O_2\)(slow)
\(H_2O_2+H_2⟶2H_2O\)(fast)
Solution:
The rate determining step is the slow step. In this case that step of the mechanism is \(2NO+H_2⟶N_2+H_2O_2\). Both \(2NO\) and \(H_2\) are reactants in the final balanced chemical equation, so there is no need to substitute for intermediates. The rate law is simply \(rate = k[NO]^2[H_2]\).
Answer:
\(rate = k[NO]^2[H_2]\).
Q21.4.20
What is the age of mummified primate skin that contains 8.25% of the original quantity of \(^1\)\(^4C\)?
Tutorial:
The equation used for radiometric dating is fraction remaining = \(0.5\)\(^t\)\(^/\)\(^h\). The t stands for the age of the object being dated and h refers to the half-life of the radioactive compound.
Solution:
In this case, the radioactive compound is \(^1\)\(^4C\) which has a half-life of 5730 years and we are solving for t.
Plugging in the equation:
\(0.0825 = 0.5\)\(^t\)\(^/\)\(^h\)
\(ln(0.0825) = (t/5730)(ln0.5)\) Take the natural log of both sides of the equation. Use log rules to put the exponent in front.
\(ln(0.0825)/(ln0.5) = (t/5730)\) Divide the left side by \((ln0.5)\) and continue solving for t
\((ln(0.0825)/(ln0.5)) x 5730 = t\) Solve for t
\(t = ~20,625 years\)
Answer:
The mummified primate skin is about 20,625 years old.
Q20.3.8
Consider the following spontaneous redox reaction: \(NO_3^-\)(aq) + \(H^+\)(aq) + \(SO_3^-2\)(aq) → \(SO_4^-2\)(aq) + \(HNO_2\)(aq).
Tutorial:
The two half-reactions that take place will be the reduction half reaction and the oxidation half reaction. The half reactions will include the balanced number of electrons that are lost or gained.
Some things to remember:
oxidation definition: loss of electrons, therefore increasing the oxidation number
reduction definition: gain of electrons, therefore decreasing the oxidation number
-the sum of the oxidation numbers in a compound is zero
-the sum of the oxidation numbers in a polyatomic ion is equal to the charge
Cathode: electrode where reduction occurs, electrons flow to cathode combining with the metal ion so metal will plate, concentration of metal ion is solution will decrease, reaction will eventually slow
Anode: electrode where oxidation occurs, metal ion and electrons formed. Since electrons are present, anode is negative
Solutions:
1. Write the two half-reactions for this overall reaction.
The reduction half reaction is the one involving the gain of electrons. The nitrogen on the reactant side has an oxidation number of +5 and on the product side has an oxidation number of +3, therefore it was reduced. The oxidation half reaction is the one involving the loss of electrons. The sulfur on the reactant side has an oxidation number of +4 and on the product side has an oxidation number of +6, therefore it was oxidized.
reduction: \(NO_3^-\)(aq) + \(H^+\)(aq) + \(2e^-\)→ \(HNO_2\)(aq)
oxidation: \(SO_3^-2\)(aq) → \(SO_4^-2\)(aq) + \(2e^-\)
2. If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which electrode corresponds to which half-reaction?
The two electrodes are the cathode and the anode. The cathode is where reduction occurs and the anode is where oxidation occurs. Therefore the reduction half reaction occurs in the cathode and the oxidation half reaction occurs in the anode.
3. Which electrode is negatively charged, and which is positively charged?
Due to the presence of electrons, the anode is negatively charged. Therefore the cathode is positve.
Answers:
1.
reduction: \(NO_3^-\)(aq) + \(H^+\)(aq) + \(2e^-\)→ \(HNO_2\)(aq)
oxidation: \(SO_3^-2\)(aq) → \(SO_4^-2\)(aq) + \(2e^-\)
2.
the reduction half reaction occurs in the cathode and the oxidation half reaction occurs in the anode
3.
cathode is positive
anode is negative
Q20.5.19
![]()
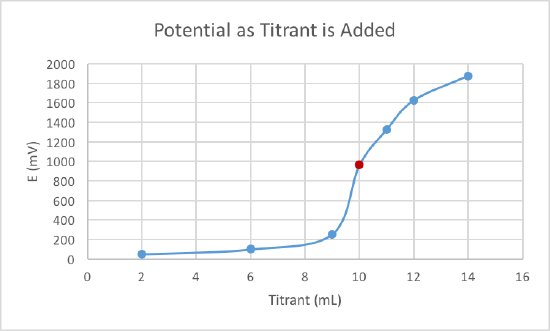
Potentiometric titrations are an efficient method for determining the endpoint of a redox titration. In such a titration, the potential of the solution is monitored as measured volumes of an oxidant or a reductant are added. Data for a typical titration, the potentiometric titration of \(Fe\)(II) with a 0.1 M solution of \(Ce\)(IV), are given in the following table. The starting potential has been arbitrarily set equal to zero because it is the change in potential with the addition of the oxidant that is important.
| Titrant (mL) | E (mV) |
|---|---|
| 2.00 | 50 |
| 6.00 | 100 |
| 9.00 | 255 |
| 10.00 | 960 |
| 11.00 | 1325 |
| 12.00 | 1625 |
| 14.00 | 1875 |
Tutorial:
A redox reaction involves one atom gaining an electron and another atom loosing an electron. The number of electrons lost by one atom is equal to the number of electrons gained by the other atom.
oxidation definition: loss of electrons, therefore increasing the oxidation number
reduction definition: gain of electrons, therefore decreasing the oxidation number
A titration curve can be used to show the equivalence and endpoint. Locate the area of exponential growth on the curve. The middle of this exponential growth is the equivalence point and the end of the exponential growth, before the upper curve, is the endpoint. At the equivalence point the moles of titrant is equal to the analyte.
Solutions:
1. Write the balanced chemical equation for the oxidation of \(Fe^+2\) by \(Ce^+4\).
The \(Fe^+2\) is being oxidized by the \(Ce^+4\). Therefore, \(Fe^+2\) will loose an electron and \(Ce^+4\) will gain an electron.
\(Fe^+2\) + \(Ce^+4\) → \(Fe^+3\) + \(Ce^+3\)
2. Plot the data and then locate the endpoint.
The end of the exponential growth, before the upper curve, is the endpoint (marked in red).
3. How many millimoles of \(Fe^+2\) did the solution being titrated originally contain?
\(0.1 moles/Liter X 0.01 L = 0.001 moles\)
\(0.001 moles\) \(Ce^+3\) \(X 1 mol\) \(Fe^+2\)/\(1 mol\) \(Ce^+3\) \(= 0.001 moles\) \(Fe^+2\)
\(0.001 moles X 1000 milimoles/mol = 1 milimole\)
Answers:
\(Fe^+2\) + \(Ce^+4\) → \(Fe^+3\) + \(Ce^+3\)

1 milimole

