Extra Credit 36
- Page ID
- 83432
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Blue text by Haley Hudson.
Q19.14A
What voltage is required for the electrolysis of the following reactions? All reactants are in standards states.
- \(\mathrm{Zn(s) + Sn^{2+}(aq) \rightarrow Zn^{2+}(aq) + Sn(s)}\)
- \(\mathrm{2Fe^{2+}(aq) + Hg^{2+}(aq) \rightarrow 2Fe^{3+}(aq) + Hg(l)}\)
- \(\mathrm{Cu(s) + Sr^{2+}(aq) \rightarrow Cu^{2+}(aq) + Sr(s)}\)
S 19.14A
a. oxidation: Zn(s) ---> Zn2+(aq) + 2e- Eocell= -0.763V
reduction: Sn2+(aq) + 2e- ---> Sn(s) Eocell: -0.137V
lose of electrons = oxidation = anode
gain of electrons = reduction= cathode
Use this equation to find Ecell:
\(\displaystyle E_0cell=E_{0cathode}-E_{0anode}\)
(Eocell cathode: -0.137V) - ( Eocell anode= -0.763V )= -.0137+ 0.763=
Zn2+(s) + Sn2+(aq) ---> Zn2+(aq) + Sn(s) Eocell: 0.626V
b. oxidation: 2Fe2+(aq) ---> 2Fe3+ + 2e- Eocell: 0.771V
reduction: Hg2+(aq) + 2e- ---> Hg(l) Eocell: 0.854V
(Eocell cathode: 0.854V) - ( Eocell anode= 0.771V )=
2Fe2+(aq) + Hg2+(aq) ---> 2Fe3+(aq) +Hg(l) Eocell:0.083V
c. oxidation: Cu(s) ---> Cu2+(aq) + 2e- Eocell: 0.337V
reduction: Sr2+(aq) + 2e- ---> Sr(s) Eocell: -2.89V
(Eocell cathode: -2.89V) - ( Eocell anode= 0.337V )=
Cu(s) + Sr2+(aq) ---> Cu2+(aq) + Sr(s) Eocell: -3.227V
Q 20.1A
Draw and write out the electronic configurations of these 6 transition metal ions
- Ti4+
- V2+
- Cu
- Mn2+
- Cr3+
S 20.1A
When taking electrons from the D-orbital, take from the 4s electrons first before removing from the D-orbitals.
a. Ti4+: [Ar]
b. V2+: [Ar]3d3
c. Cu: [Ar]3d104s1
d. Mn2+: [Ar]3d5
e. Cr3+: [Ar]3d3
Q 21.9B
Draw the following structures.
- pentamminechlorocobalt(III)ion
- dioxalatonickel(II) ion
- diamminetrichloronitrito-N-chromium(III) ion
S 21.9B

Q24.17B
A first order reaction \(\ce{A \rightarrow products}\) has a half life of 120 seconds calculate the following:
- What percentage of \(\ce{A}\) remains unreacted after 800 seconds of reaction.
- What is the rate of reaction at \(\mathrm{[A] = 0.25\, M}\)?
S24.17B
a. t1/2=ln2/k
k=ln2/120
=5.77 x 10-3
[A]t/[A]o= percent unreacted
=e-kt
=e(-0.00577(800))
=0.98% unreacted
b. rate=k[c]
=0.00577(0.25)
=0.001443 M/s
For more help see: The Rate Law
Use this equation to for a first order reaction to first find K.
- \(\mathrm{t_{1/2} (first\: order) = \dfrac{\ln 2}{k}}\)
\(\mathrm{k = \dfrac{\ln 2}{t_{1/2}} = \dfrac{\ln 2}{120\: s} = 5.77\times10^{-3}}\)
Then plug in K and T(time)
percent unreacted defined by \(\mathrm{\dfrac{[A]_t}{[A]_0}}\)
\(\begin{align}
\mathrm{\dfrac{[A]_t}{[A]_0}} &= \mathrm{e^{-kt}}\\
&= \mathrm{e^{\large{(-5.77\times10^{-3}\times800)}} \times 100 = 0.98\% \:remains\: unreached.}
\end{align}\) - For a first order reaction, \(\mathrm{rate = k[C]}\)
\(\mathrm{= 0.0046/s \times 0.25 }\)
\(\mathrm{= 1.15\times10^{-3}\, M/s}\)
Q25.1C
- What nuclide results from alpha decay of \(\ce{^{232}Pa}\)?
- What nuclide results from β- decay of \(\ce{^{243}Am}\)?
S25.1C
Check out Figure 5.3.1 for different kinds of nuclear decay reactions and equations.
Use this equation for alpha particle emission
\(\mathrm{_{Z}^{A}{X} \rightarrow _{A-4}^{Z-2}X^{'}\ +_{2}^{4}\alpha}\)
a. 232Pa ---> 228Ac + 4He
Use this equation for Beta particle emission
\(\mathrm{_{Z}^{A}{X} \rightarrow _{Z+1}^{A}X^{'}\ +_{-1}^{0}\beta}\)
b. 243Am ---> 243Cm + -1B
Q25.33F
Determine the energy of the destruction of 1.00g of matter.
S25.33F
E=mc2
=1g x (1kg/1000g) x (3x108)2
= 9.00 x 1013 J
Use the mass energy equivalence to find the Energy released from the full conversion of 1.00g of matter.
1.Convert grams to kilograms (because Joules are Kg/m2/s-1)
2. multiply by C2 (the speed of light)
\(\mathrm{E=mc^2}\)
\(\mathrm{E = 1.0\, g \times \left(\dfrac{1\,kg}{1000\, g}\right) \times (3.00\times10^8)^2 = 9.00 \times 10^{13}\, kg\, m^2\, s^{-2} = 9.00 \times 10^{13}\, J}\)
Q21.2.4
The stability of a nucleus can be described using two values. What are they, and how do they differ from each other?
S21.2.4
The overall number of nucleons and the overall number of protons determine the stability of the nucleus. The number of nucleons is the number of protons and neutrons combined. They are different from each other because the number of nucleons includes protons and neutrons but the number of protons only includes protons.
What determines Stability:
- The neutron : proton ratio, whether a given isotope will decay by beta decay or positron decay based on the Belt of Stability. Above Z = 20 the number of neutrons always exceeds the number of protons in stable isotopes.

- The nuclear binding energies of given nuclei. Binding energies are the energy released from breaking up the nucleus into its separate nucleons or as the energy released when the nucleus is formed from separate nucleons.These can be calculated with the equation E=mc2 . M being the mass, and c the speed of light. The stronger the binding energy for a compound, the more stable it is and vis versa.
Q20.3.2
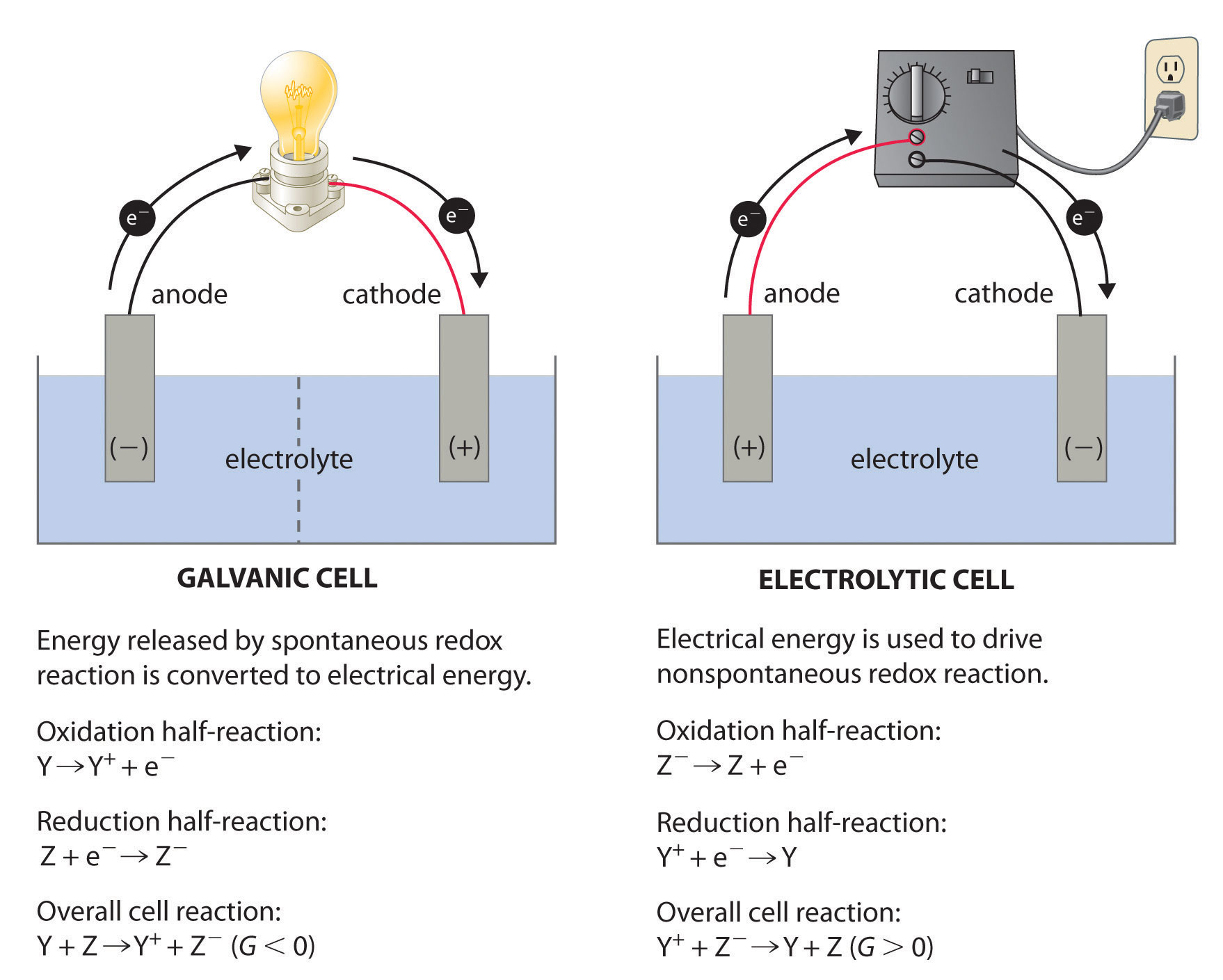
If two half-reactions are physically separated, how is it possible for a redox reaction to occur? What is the name of the apparatus in which two half-reactions are carried out simultaneously?
S20.3.2
If two half-reactions are physically separated, it is possible for a redox reaction to occur because an external electrical connection forms a circuit that allows the electrons flow from the oxidation reaction to the reduction reaction. The name of the apparatus in which two half-reactions are carried out simultaneously is called the electrochemical cell (Galvanic cell). It can also occur in a electrolytic cell which is driven by electrical energy to moving in a nonspontaneous direction while electrochemical cells connect spontaneous reactions. These cells must be neutralized by a salt bridge for electrons to continue to flow.