Extra Credit 15
- Page ID
- 82720
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q 17.2.4
In order to balance the following equations, one must noticed that all of them are Redox reactions. Thus, the half-reaction method must be used to balance each equation.
Note
One must be familiar with the rules of how to assign oxidation states to atoms within an equation. These rules can be found here in detail: Oxidation states
Examples For Balancing Equations:
A) Al(s) + Zr4+(aq) → Al3+(aq) + Zr(s)
To balance this equation, we must split it into its two half reactions; the oxidation half reaction and the reduction half reaction.
Oxidation Half Reaction: Al(s) → Al3+(aq) (This is the oxidation half reaction because the oxidation state of Al is going from 0 to +3)
Reduction Half Reaction: Zr4+(aq) → Zr(s) (This is the reduction half reaction because the oxidation state of Zr goes from 4+ to 0)
Next, for each half reaction, we must balance all of the atoms that are no H or O. These are already balanced so we can leave them alone. There are also no H or O atoms to balance next which makes our job simpler. The next thing to balance is the charges for each equation. As we can see in the oxidation half reaction, the reactant side has a 0 charge while the product side has a charge of 3+. Thus we add three electrons to the reactant side yielding: Al(s) → 3e- + Al3+(aq). We also do a similar process with the reduction half reaction resulting in: Zr4+(aq)+ 4e- → Zr(s).
Since the electrons that are transferred in each equation are different from each other we must make sure each equation is transferring the same amount of electrons before they can be added together. So for the oxidation half reaction we will multiple the whole equation by 4 and for the reduction half reaction we will multiple everything by 3. This will result in the balanced equation:
4Al(s)+3Zr4+ → 4Al3+(aq) + 3Zr(s)
B) Ag+(aq) + NO(g) → Ag(s) + NO3-(aq) (acidic solution)
Make note that we need to balance this equation under acidic solution, this will affect how we balance the equation.
To start off, we will split the equation into its two half reactions.
Oxidation Half Reaction: NO(g) → NO3-(aq) (N goes from 2+ to 5+)
Reduction Half Reaction: Ag+(aq) → Ag(s) (Ag goes from +1 to 0)
Again, we first must balance all atoms besides O and H. Those atoms are already balanced for us. Next, since we are in acidic solution, we balance out the O by adding H2O to the other side of the equation. Then to balance out the H's, add H+ to the other side of the equation. We are able to do this since this reaction is occurring under acidic solution. This gives us these two equations:
NO(g) + 3H2O(l) → NO3-(aq) + 6H+(aq)
Ag+(aq) → Ag(s)
Next we have to balance out the charges by adding electrons to the more positive side of the equation until the charges are balanced. We then get these two equations:
NO(g) + 3H2O(l) → NO3-(aq) + 6H+(aq) + 5e-
Ag+(aq) + e- → Ag(s)
Once again, the electrons transferred in each half reaction are different from each other so we need to make sure they are equal. We will multiple the reduction half reaction by 5 so that each equation is transferring 5 electrons. This gives us the balanced equation:
5Ag+(aq) + NO(g) + 3H2O → 5Ag(s) + NO3-(aq) + 6H+(aq)
C) SiO32-(aq) + Mg(s) → Si(s) + Mg(OH)2 (s) (basic solution)
Note: We are balancing this equation under basic conditions.
First, write down the two half reactions:
Oxidation Half Reaction: Mg(s) → Mg(OH)2 (s) (Mg goes from 0 to +2)
Reduction Half Reaction: SiO32-(aq) → Si(s) (Si goes from +4 to 0)
We do not need to balance Si and Mg since they are already balanced for us, so we will proceed to balance the oxygen and hydrogen atoms. Since we are in basic solution, when we add H+ we need to neutralize it with OH- by adding it to both sides. By doing this process, we get the following equations:
Mg(s) + 2H2O(l) + 2OH-(aq) → Mg(OH)2 (s) + 2H+(aq) + 2OH-(aq)
SiO32-(aq) + 6H+(aq) + 6OH-(aq) → Si(s) + 3H2O(l) + 6OH-(aq)
Now we need to combine the H+ and OH- to form water:
Mg(s) + 2H2O(l) + 2OH-(aq) → Mg(OH)2 (s) + 2H2O(l)
SiO32-(aq) + 6H2O(l) → Si(s) + 3H2O(l) + 6OH-(aq)
Cancel out the waters from each side of the equation:
Mg(s) + 2OH-(aq) → Mg(OH)2 (s)
SiO32-(aq) + 3H2O(l) → Si(s) + 6OH-(aq)
Now balance out the charges with the addition of electrons.
Mg(s) + 2OH-(aq) → Mg(OH)2 (s) + 2e-
4e- + SiO32-(aq) + 3H2O(l) → Si(s) + 6OH-(aq)
Multiple the top equation by 2 so that both half reactions are transferring 4 electrons. Now they can be added together to get:
SiO32-(aq) + 2Mg(s) + 3H2O(l) → Si(s) + 2Mg(OH)2 (s) + 2OH-(aq)
D) ClO3-(aq) + MnO2 (s) → Cl-(aq) + MnO4-(aq) (basic solution)
First, write down the following half reactions:
Oxidation Half Reaction: MnO2 (s) → MnO4-(aq) (Mn goes from +2 to +7)
Reduction Half Reaction: ClO3-(aq) → Cl-(aq) (Cl goes from +5 to -1)
Once again, the other atoms (besides H and O) are already balanced in the given equation. Now we must balance the O and H by adding water and H+ respectively to attain the following equations:
MnO2 (s) + 2H2O(l) → MnO4-(aq) + 4H+(aq)
ClO3-(aq) + 6H+(aq) → Cl-(aq) + 3H2O(l)
Because we are under basic conditions we must perform similar steps as in problem C. We will add OH- to neutralize the H+ into water giving us these equations:
MnO2 (s) + 2H2O(l) + 4OH-(aq) → MnO4-(aq) + 4H2O(l)
ClO3-(aq) + 6H2O(l) → Cl-(aq) + 3H2O(l) + 6OH-(aq)
Then cancel out the waters to attain these equations:
MnO2 (s) + 4OH-(aq) → MnO4-(aq) + 2H2O(l)
ClO3-(aq) + 3H2O(l) → Cl-(aq) + 6OH-(aq)
Add electrons to balance out charges:
MnO2 (s) + 4OH-(aq) → MnO4-(aq) + 2H2O(l) + 3e-
ClO3-(aq) + 3H2O(l) + 6e- → Cl-(aq) + 6OH-(aq)
To make sure both equations are transferring the same amount of electrons, multiple the top equation by 2 so that both half reactions are involving 6 electrons. Add the two half reactions together to get:
ClO3-(aq) + 2MnO2 (s) + 2OH-(aq) → Cl-(aq) + 2MnO4-(aq) + H2O
Example Cell Notation:
Note: For cell notation the anode (oxidation half reaction) is always placed on the left side while the cathode (reduction half reaction) is placed on the right side of the cell notation. A | means that there is a phase change. A || means that there is a salt bridge placed there in order to allow the cell to keep its electrical neutrality and separate the anode from the cathode. Stoichiometry is not included in the cell notation.
A) Al(s) + Zr4+(aq) → Al3+(aq) + Zr(s)
We have already identified in the previous example the half reactions of this equation, and therefore we already know the anode and the cathode. This allows us to create a cell notation that looks like this:
Al(s)|Al3+(aq)||Zr4+(aq)|Zr(s)
The solid aluminum and Zr act as electrodes for this cell. The oxidation half reaction is placed on the left side and the reduction half reaction is on the right. This a relatively simple cell diagram to set up.
B) Ag+(aq) + NO(g) → Ag(s) + NO3-(aq)
We already have the two half reactions available to us and therefore we can create a cell notation that looks like this:
Pt(s)|NO(g)|NO3-(aq)||Ag+(aq)|Ag(s)
Noticed for the oxidation half reaction there was no solid to use as an electrode, only a gas and a solution. That is why we introduce platinum (Pt) as an inert electrode. It does not partake in the redox reaction itself, it merely acts as a source of electrons.
C) SiO32-(aq) + Mg(s) → Si(s) + Mg(OH)2 (s)
Again we have the half reactions available to us from the examples explaining how to balance the equations. The cell notation should look like:
Mg(s), Mg(OH)2 (s)|OH-(aq)||SiO32-(aq)|Si(s)
Here, when there is no phase change, as seen at the anode, which is signified using a comma. The OH- solution must be added to the cell notion to show that the reaction is occurring in an aqueous environment. The cathode side of the cell notation follows the basic rules.
D) ClO3-(aq) + MnO2 (s) → Cl-(aq) + MnO4-(aq)
As with the other problems, we know the half reactions already. Therefore we create the cell notation:
MnO2 (s)|MnO4-(aq)||ClO3-(aq), Cl-(aq)|Pt(s)
Here, we once again see a comma in the cathode since ClO3- and Cl- both are in the same phase. Because of this, we need to add an inert electrode in order to have something to connect a wire too in order to measure the cell potential. We can again use platinum as the inert electrode since it does not participate in the redox reaction.
Q 19.1.13
For a the table of standard reduction potentials to use in this problem visit this link: Reduction Potential Table
Example:
Find the potentials of the following electrochemical cell:
Cd | Cd2+ (M = 0.10) ‖ Ni2+ (M = 0.50) | Ni
This cell notation describes a reaction that takes place at nonstandard conditions. At standard conditions, the molarity would be at 1.0M for each but it is not for this example. Therefore we will have to make use to the Nernest Equation: E =E°- (RT/nF)(lnQ) where E° is the standard reduction potential at standard conditions, F stands for Faraday's constant, R a gas constant, and n is the number of moles of electrons that is transferred. We can assume this reaction happens at room temperature (298K) we can simplify the Nernest Equation to: E =E° - (0.0592V/n)(logQ). First, we will must balance the equation in order to determine the number of moles of electrons transferred. This is a redox reaction so we will follow similar rules as in Q17.2.4. When we split the equation into two half reactions and add the electrons transferred we get:
Oxidation Half Reaction: Cd(s) → Cd2+(aq) + 2e-
Reduction Half Reaction: Ni2+(aq) + 2e- → Ni(s)
From this we see that 2 electrons are transferred in the cell, thus n=2. This gives us the equation: E =E° - (0.0592V/2)(logQ).
In order to get E° we will use the equation E°= cathode - anode, where we use the standard reduction potentials from the table and subtract it from each other. This will give us the cell potential under standard conditions. Using the link above to access the table we can solve for it:
E°= -0.25V - (-0.403V)= 0.153V
Now we need to figure out the Q constant. This can be determined by placing the molarity of the products and divide them by the molarity of the reactants. Note that we ignore solids in this since there concentration does not change with time. Thus, we get:
Q= [Cd2+]/[Ni2+] = [0.10]/[0.50] = 0.20
So now we can plug in all of our numbers into the simplified Nernest equation to solve this problem.
E = 0.153V - (0.0592V/2)(log 0.020) = 0.153V - (-0.0207V) = 0.174V
So under these nonstandard conditions, the cell potential is 0.174V compared to 0.153V at standard potentials.
Q 19.3.5
Is it possible for a complex of a metal in the transition series to have six unpaired electrons? Explain.
It is not possible for a metal in the transition series to have six unpaired electrons. This is because transition metals have a general electron configuration of (n-1)d1-10 ns1-2 where n is the quantum number. The last electron will go into the d orbital which has 5 orbitals that can each contain 2 electrons, yielding 10 electrons total. According to Hund's Rule, electrons prefer to fill each orbital singly before they pair up. This is more energetically favorable. Since there are only 5 orbitals and due to Hund's Rule, the maximum number of unpaired electrons a transition metal can have is 5. Therefore, there cannot be a complex of a transition metal that has 6 unpaired electrons.
For example, lets look at iron's electron configuration. Iron has an electron configuration of 1s22s22p63s23p64s23d6. Now the most important orbital to look at is the d orbital which has 6 electrons in it, but there are only 4 unpaired electrons as you can see by this diagram:
3d: [↿⇂] [↿] [↿][↿][↿]
Each [ ] represents an orbital within the d orbital. This diagram follows Hund's rule and shows why no transition metal can have 46 unpaired electrons.
Q 12.4.5
From the given data, use a graphical method to determine the order and rate constant of the following reaction:
2X⟶Y+Z
| Time (s) | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 |
|---|---|---|---|---|---|---|---|---|
| [X] (M) | 0.0990 | 0.0497 | 0.0332 | 0.0249 | 0.0200 | 0.0166 | 0.0143 | 0.0125 |
In order to determine the order of the reaction we need to plot the data using three different graphs. All three graphs will have time in seconds as the x-axis, but the y-axis is what will differ. One graph will plot concentration versus time, the second will plot natural log of concentration versus time, and the other will plot 1/concentration versus times. Whichever graph results in a line, we know that must be the order of the reaction. If we get a line using the first graph, it will be zero order, if it is a line for the second graph it will be first order, and if it is a line for the third graph it will be a second order reaction. Now lets plot the data to determine the order.
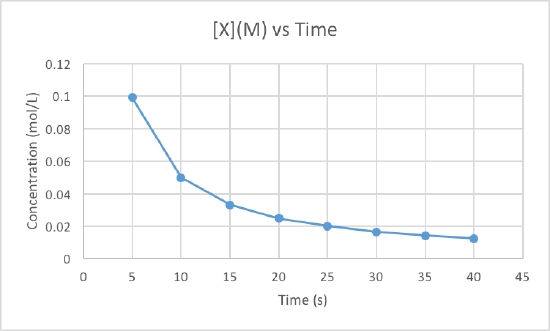
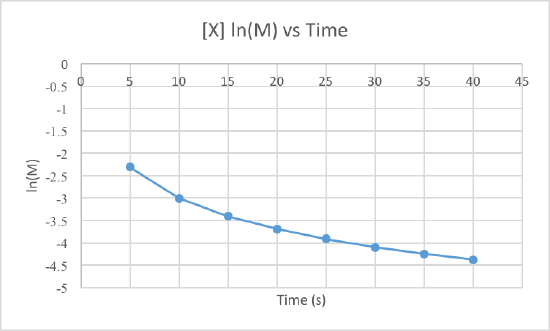
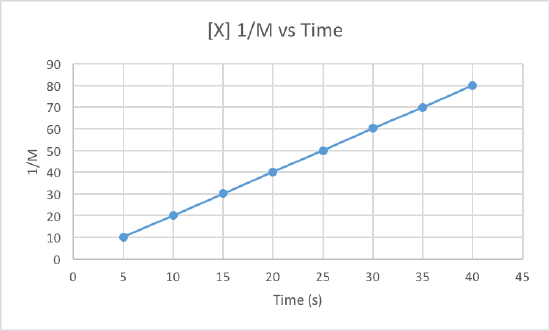
We can clearly see that the third graph, which plots 1/M versus time, is a straight line while the other two are slightly curved. Therefore, we can determine that the rate of this reaction is second order. This also tells us that the units of the rate constant which should be M-2s-1 for a second order reaction.
To determine the rate constant, called k, we simple need to figure out the slope of the third graph since that is the order of this reaction. To find the slope of the line, we take two points and subtract the y values and then divide them by the difference of the x values. This is how to do it:
Use the points (5, 10.101) and (40, 80).
Now use these to get the slop, aka the rate constant: (80-10.101)/(40-5) = 1.997 = k
So the rate constant for this second order reaction is 1.997 M-1s-1.
Q 12.7.8
a)
b)
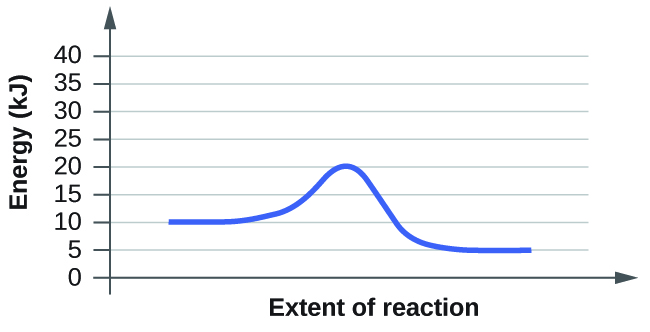
c)
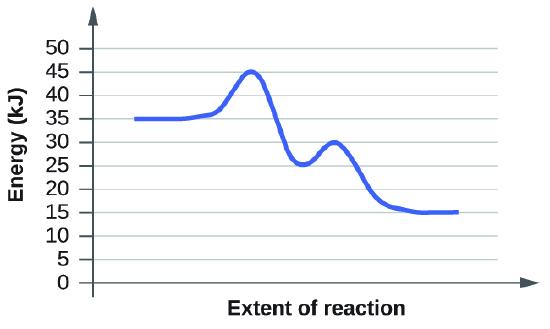
d)
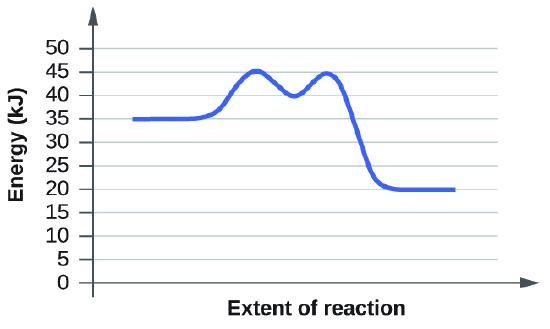
Example 1:
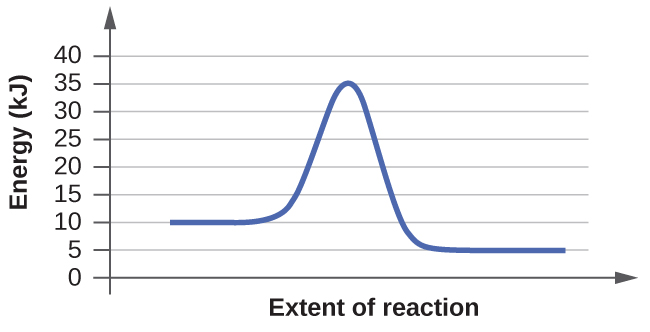
Based on the diagrams a and b, which of the reactions has the fastest rate? Which has the slowest rate?
The rate of a reaction is determined by its slowest step. What makes a step in a reaction slow has to do with its activation energy. Activation energy is the minimum amount of energy required by the reactants in a reaction to undergo a specific reaction. If the reactants do not have the required energy than the reaction will not proceed. With this in mind, we can see that B will have the fastest rate while A has the slowest out of the two. B has the faster rate since it has a smaller activation energy compared to A. The reactants if B need less energy to become products and thus can proceed at a faster rate than the reactants of A. To see this numerical, you take the highest energy needed and subtract it from the energy of the reactants to see how much energy is still needed. For B it is 20kJ-10kJ= 10kJ for the activation energy. For A it is 35kJ - 10kJ= 25kJ for the activation energy. 25kJ is greater than 10kJ so A will proceed the slowest since its reactants need to attain more energy in order to get the reaction going.
Example 2:
Based on the diagrams c and d, which of the reactions has the fastest rate? Which has the slowest rate?
Here the rules of activation energy as a barrier still apply, but in these two diagrams we now see intermediate steps with two activation energies needed. However, the rate of a reaction is only determined by its slowest step. If you look at the two diagrams you will see that both of them have the same first activation energy required: 45kJ - 35kJ = 10kJ. And this is the slowest step in both reactions. In diagram c, the second activation energy is much lower than the second activation energy in diagram, but these do not determine the rate of the reaction. Since the slowest step for both reactions is the same, they each will have the same rate as the other.
Q 21.5.3
Both fusion and fission are nuclear reactions. Why is a very high temperature required for fusion, but not for fission?
Definition
Fission is the splitting of a heavy nucleus into lighter nuclei. This can often be artificially induced by bombarding a stable isotope with neutrons to make it unstable so that is will split into two large fragments.
Fusion is the process of combining lighter nuclei to create a heavier nucleus. If the fusion occurs with two isotopes lighter than iron, the reaction will release energy, if it is with isotopes heavier than iron it will absorb energy. This process occurs in stars, such as our sun.
Fission does not require high temperatures in order to split a nucleus because the nucleus of the isotope is unstable. Just by bombarding it with high speed particles will cause the isotope to split into more stable species. This process will start a chain reaction that happens spontaneously until a very stable atom is finally reached. This process does not require extremely high temperatures. Fusion, on the other hand, does. It requires extremely high temperatures because it is forcing two light nuclei to form a heavier element. A nucleus contains protons and neutrons, with protons beings positively charged and neutrons having no charge. To force two nuclei together, you must overcome the stronger repellent electrostatic forces due to the like positive charges. This repulsion is why it requires such high temperature for fusion to occur. That is why the sun is an ideal place for fusion to take place.
Q 20.4.1
Is a hydrogen electrode chemically inert? What is the major disadvantage to using a hydrogen electrode?
Definition
Electrode is a point where current enters and leaves the electrolyte. It is a solid (often a metal) where the oxidation or reduction reaction occurs between itself and the surrounding solution.
Inert Electrode is an electrode that is not made of the substance being oxidized or reduced. It is often a good conductor as it can transfer electrons but it does not undergo a redox reaction itself
The hydrogen electrode is chemically inert because in the standard hydrogen electrode, the hydrogen gas is at 1 bar pressure which is passed into 1M H+ at 298K in which a platinum electrode is immersed, as it is chemically unreactive and under this condition it only allows the inflow or outflow of electrons without reacting. Therefore, the hydrogen electrode is inert since it is using platinum which is a common inert electrode. Also, the platinum is not made of the substance being oxidized or reduced which once again shows that the hydrogen electrode is chemically inert.
The major disadvantage of using hydrogen electrode is its cost because platinum as well as hydrogen is very expensive element. Hydrogen must be constantly supplied to the standard hydrogen electrode which keeps adding to the cost. So it is very expensive to use standard hydrogen electrode in labs. Also, it is a difficult electrode to set up so it is often considered a hypothetical electrode because of this.
Q 20.5.30
Example:
Hydrogen gas reduces Ni2+ according to the following reaction: Ni2+(aq) + H2 (g) → Ni(s) + 2H+(aq); E°cell = −0.25 V; ΔH = 54 kJ/mol.
- What is K for this redox reaction?
- Is this reaction likely to occur?
- What conditions can be changed to increase the likelihood that the reaction will occur as written?
- Is the reaction more likely to occur at higher or lower pH?
We will first try to tackle questions 1 and 2 at the same time. To find out if the reaction is likely to occur, we must find the delta G°. If it is positive, then the reaction will be nonspontaneous, but if it is negative it will be spontaneous. In order to find the delta G° we must recall the equation: ΔG° = -nFE°
n is the number of moles of electrons transferred, F is faraday's constant which is 96500 C/mol, and E° is the standard cell potential for the given cell. We need to find n so we must split the reaction into its two half reactions.
Reduction Half Reaction: Ni2+(aq) + 2e- → Ni(s)
Oxidation Half Reaction: H2 (g) → 2H+(aq) +2e-(aq)
So this allows us to determine that n=2. Eº is already given in the question as -0.25V so we can now plug into the equation above:
ΔG° = -(2)(96486 C/mol e-)(-0.25) = 48243 J/mol
Now to solve for K (equilibrium constant) we will use the equation: ΔG° = -RTlnK where R is the gas constant 8.3145 JK-1mol-1
Since the problem does not mention a specific temperture, we will assume the reaction takes place at room temeprture or 298K. Now we will solve for K:
48243 J/mol = -(8.3145JK-1mol-1)(298K)lnK
48243 J/mol = (-2477.721 mol-1)lnK
-19.471 = lnK
Then take the e of both sides to get: 3.498x10-9 (equilibrium constants do not have constants)
Now we can use this information to solve problems 1-4.
- Answer:
-
- The K for this redox reaction is: K= 3.498x10-9
- Since the ΔG° is equal to 48243 J/mol, which is a positive number, it shows that this reaction is nonspontaneous and therefore is unlikely to occur. Also the K value is extremely small which means the forward reaction is not favored which once again shows that the reaction is not likely to occur.
- The ΔH = 54 kJ/mol which is an endothermic reaction. That means it takes energy or heat to make the reaction happen. In an endothermic reaction, heat acts as a reactant and therefore if we increase the temperature then the forward reactions and the products will be favored. This can be concluded due too le chatelier's principle.
- pH shows us the level of H+ ions within a solution. The lower the pH the more H+ in the solution and therefore its more acidic. The higher the pH, the less H+ in the solution and therefore its more basic. Once again, using le chatelier's principles, we can increase the forward reaction by either increasing the reactants or taking away some of the products. H+ is a product of this redox reaction, therefore if we want to make the forward reaction more favorable, we should lower this amount. Therefore, we will want this reaction to occur at lower pH levels in order to make this reaction more likely to occur.

