Extra Credit 25
- Page ID
- 83534
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Edited by Jodie Stroeve
Q19.8B
For the reaction
\(\mathrm{Cr_2O_7^{2-}+(aq)+6Ag(s)→2Cr^{3+}(aq) +6Ag^+(aq)+7H_2O(l)}\)
Use the data table to determine
- Eºcell
- K
- ΔGº
- Whether the reaction goes substantially to completion when the reactants and products are initially in their standard state.
S19.8B
To determine the Eºcell of this reaction, we first need to know the reduction potentials of each half-reaction. These can be found in the reduction potential tower.
- Half reactions:
\(\mathrm{Cathode: {Cr_2O_7^{2-}+14H^++6e^- →2Cr^{3+}+H_2O}}\) \(\mathrm{E=+1.33V}\) According to Table P2: Standard Reduction Potentials Table, your cathode half-cell reaction is incorrect in the number of moles of water produced. It should be seven moles of water transferred rather than one. Balancing the elements in this equation will confirm that the number of atoms of water and oxygen on the reactants vs. products side do not add up if the coefficient in front of water is one, but does equate if the number of moles created is seven.
2)according to the P2: Standard Reductions Table on Libre Texts, the half-cell reduction potential value for this reaction is 1.36 V. I do not think your half cell standard reduction value is completely incorrect, but it may not be as precise as the ones provided through Libretexts.
\(\mathrm{Anode: {Ag(s)→Ag^++e^-}}\) \(\mathrm{E=0.800V}\)
1) The same goes for this half-reaction. According to Table P2: Standard Reduction Potentials Table, the value of the standard cell potential for the reduction half-reaction is .7996 Volts. This is very very close to the value you have, so it is up to you on whether or not to change it, but I do believe that this table provides more accurate reduction values.
Then, the equation for standard cell potential can be applied with the reduction potential values at the cathode and anode.
\(\mathrm{E_{cell}=E_{cathode}-E_{anode}}\)
\(\mathrm{=1.33-0.800V}\)
\(\mathrm{=0.53V}\)
The calculated standard cell potential using the new values of the standard cell reduction potentials for both the anode and the cathode would be .5604 Volts (.560 with correct number of significant digits).
- When standard cell potential has been calculated, the free gibbs energy can also be calculated using an equation that relates the two variables together.
Standard conditions:
\(\mathrm{ΔGº=-nFEº_{cell}}\)
\(\mathrm{=(-6mol)(96485Cmol^{-1})(0.53V)}\)
\(\mathrm{=-30682.23V}\)
1) The units of Gibb's free energy are not volts, but rather joules because they are concerning energy, not electrical potential. To further support this, I J = I V * I C confirms that the units of energy of the product in this equation should be in Joules since the remaining units are in V*C.
2) If you use the calculated standard cell potential using the new values of the standard cell reduction potentials for both the anode and the cathode, the Gibb's free energy would be 324421.164 Joules (324000 Joules with correct amount of significant digits).
A negative free gibbs energy means the reaction is spontaneous.
3. The rate constant can then be found using the equation that relates cell potential and the rate constant.
\(\mathrm{E_{cell}={(-RT/nF)}lnK}\)
\(\mathrm{K=e^{(-nFE_{cell})/(RT)}}\)
\(\mathrm{K=6.43*10^{54}}\)
1) Make sure to add in notation that you are using the cell potential of the cell under standard conditions in this equation, because it appears as if you are using the numerical value of the cell under non-standard conditions
2) The correct equation does not have a negative sign in front of the raised expression, so the correct equation would be:
\(\mathrm{E^{\circ}={(RT/nF)}lnK}\)
2) Using the new value for the standard cell reduction potentials for both the anode and the cathode that gives a standard cell potential of .5604 Volts, the rate constant would come out to \(\mathrm{K=7.38*10^{56}}\)
- The reaction can be spontaneous. ΔGº is very negative.
Because free gibbs energy is negative, this means the reaction will go to completion.
1) The value of the rate constant what tells us whether the reaction will go substantially to completion. The gibbs free energy tells us about the spontaneity of the reaction, but not whether it goes to completion.
Q19.65B
Which of the following reactions are spontaneous under standard conditions? If the reaction is not spontaneous, determine its minimum voltage applied to undergo electrolysis.
- \(\mathrm{Sn^{2+}(aq)+Zn^{2+}(aq)→Zn(s)+Sn^{4+}(aq)}\)
- \(\mathrm{Li^+(aq)+Fe^{2+}(aq)→Li(s)+Fe^{3+}(aq)}\)
- \(\mathrm{2H_2(g)+O_2(g)[in 1M H^+(aq)]→2H_2O(l)}\)
- \(\mathrm{2Al^{3+}(aq)+3Pb(s)→3Pb^{2+}(aq)+2Al(s)}\)
S19.65B
The spontaneity of the reactions below can be found by calculating the standard cell potential. This is found by looking up the reduction potential of each half-reaction then applying them to the equation that calculates for standard cell potential. When the cell potential is positive, the reaction is positive and does not need external voltage to undergo electrolysis. If the cell potential is negative, the reaction is not spontaneous and external voltage needs to be applied for the reaction to undergo electrolysis. The minimum voltage should equal the amount of voltage needed for the negative cell potential to reach 0V.
- \(\mathrm{Anode: {Sn^{2+}(aq) →Sn^{4+}(aq)+2e^-}}\) \(\mathrm{Eº_{cell}=+0.154V}\)
\(\mathrm{Cathode: {Zn^{2+}(aq)+2e^-→Zn(s)}}\) \(\mathrm{Eº_{cell}=-0.763V}\)
\(\mathrm{Eº_{cell}=−0.763V−(+0.154V)=−0.917V}\)
Minimum voltage needed to be applied to undergo electrolysis =+0.917V
- \(\mathrm{Anode: {Fe^{2+}(aq) →Fe^{3+}(aq)+e^-}}\) \(\mathrm{Eº_{cell}=+0.771V}\)
\(\mathrm{Cathode: {Li^{+}(aq)+e^-→Li(s)}}\) \(\mathrm{Eº_{cell}=-3.040V}\)
\(\mathrm{Eº_{cell}=−3.040V−(+0.771V)=−3.811V}\)
Minimum voltage needed to be applied to undergo electrolysis =+3.811V
3. \(\mathrm{Anode: {2H_2(g) →4H^+(aq)+4e^-}}\) \(\mathrm{Eº_{cell}=+0.000V}\)
\(\mathrm{Cathode: {O_2(g)+4H^+(aq)+4e^-→2H_2O(l)}}\) \(\mathrm{Eº_{cell}=+1.229V}\)
\(\mathrm{Eº_{cell}=+1.229V−(+0.000V)=+1.229V}\)
The reaction is spontaneous. No need undergo electrolysis.
4. \(\mathrm{Anode: {Pb(s) →Pb^{2+}(aq)+2e^-}}\) \(\mathrm{Eº_{cell}=-0.125V}\)
\(\mathrm{Cathode: {Al^{3+}(aq)+3e^-→Al(s)}}\) \(\mathrm{Eº_{cell}=-1.676V}\)
\(\mathrm{Eº_{cell}=−1.676V−(-0.125V)=−1.551V}\)
Minimum voltage needed to be applied to undergo electrolysis =+1.551V
You detail the anode and cathode half-cell reactions with balanced coefficients in all parts except for (d). To make it less confusing, I would label all the half cell-reactions with balanced coefficients (and specify that multiplying the non-balanced half-cell reactions by a coefficient to balance the electrons transferred does not affect the standard cell potential of the reaction). Also, maybe specify the website from which you are getting the standard cell potential values if you don't use the Table P2: Standard Reduction Potentials Table.
Q21.5B
- Use crystal field theory to draw the most probably structure of [CoF6]3- in a weak field.
- Determine whether [Cu(H2O)4]2+ is paramagnetic or diamagnetic.
S21.5B
The ligand F- is on the lower end of the spectrochemical series so it will be a weak field ligand. This mean that the ligand will induce a lower splitting of the d-orbitals, causing the electrons to be in high spin. Co usually has 9 electrons in its outer shell but because the complex has a charge of -3, Co is determined to be Co3+. This means the d-orbital will have 6 electrons. All the orbitals will be first filled in with an electron and the left over electron will be added to the first orbtial creating this crystal field diagram:
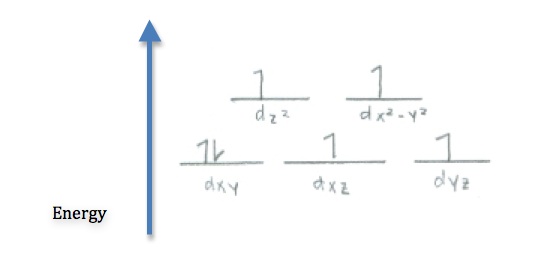
- Since Cu2+ has 9 electrons, the only way to arrange that in 5d orbitals is to have one unpaired electron. Therefore it is paramagnetic.
Maybe add a few more details as to why a weak field ligand induces a high spin complex (like how the octahedral crystal field splitting energy compares to the pairing energy and how this leads electrons to go into the higher/lower orbitals). Also, maybe describe the way in which you fill the shells differently (like saying to add one electron to each orbital first, then add a second electron to each... rather than saying left over).
Q24.13B
The following rates of reaction were obtained in three experiments with the reaction 2NO(g) + Cl2(g) —> 2NOCl (g).
|
Expt |
Initial [NO], M |
Initial [Cl2], M |
Initial Rate of Reaction, M s-1 |
|---|---|---|---|
|
1 |
0.0125 |
0.0255 |
2.23 x 10-5 |
|
2 |
0.0125 |
0.0510 |
4.46 x 10-5 |
|
3 |
0.0250 |
0.0255 |
8.92 x 10-5 |
What is the rate law of this reaction?
S24.13B
The rate law can be found by determining the rate orders for each compound within the reaction. We can find the rate order of NO by holding the concentration of Cl2 constant and calculating the change in the rate of reaction. The same process can be done with Cl2. Then the rate constant can be found by applying any one of the trials to obtain the value of the rate constant. Then all the values can be put together to obtain the rate law. The units for the rate constant can also be found by knowing the units of the rate and the concentrations of NO and Cl2 and using algebra to solve for the units of k.
\(\mathrm{NO: (0.0250/0.0125)^x=((8.92*10^{-5})/(2.23*10^{-5})}\)
\(\mathrm{=2^x=4}\)
\(\mathrm{x=2, [NO]^2}\)
\(\mathrm{CL_2: (0.0510/0.0255)^x=((4.46*10^{-5})/(2.23*10^{-5})}\)
\(\mathrm{=2^x=2}\)
\(\mathrm{x=1, [Cl_2]^1}\)
\(\mathrm{k: Rate=k[NO^2[Cl_2]^1}\)
\(\mathrm{k: 2.23*10^{-5}=k[0.0125]^2[0.0255]^1}\)
\(\mathrm{k=5.597M^2sec}\)
\(\mathrm{Rate=5.597[NO]^2[Cl_2]^1}\)
The units of the rate constant should be in \(\mathrm{k=5.597M^-2sec^-1}\) as this gives the correct overall units of M/sec for the rate of reaction
Q24.60A
The following substrate's concentration [S] versus time data were obtained during an enzyme-catalyzed reaction: t=0min; [S]=1.00M; 30min, 0.90M; 90min, 0.70M; 120min, 0.50M; 180min, 0.20M. What order is this reaction with respect to S in the concentration?
S24.60A
The order of the reaction can be found by calculating the relationship between the time and the concentration of [S]. At 0 minutes, the concentration is 1M, then at 30 minutes, the concentration is 0.9M indicating that the change in molarity over the change in time is 0.1M/30min. It is the same for the next change in molarity over the next change in time, being: 0.2M/60min which is equal to 0.1M/30min. This means the order of reaction is 2, which can be written as [S]2.
The order of the reaction can be determined by plotting the respective concentration versus time graphs for zeroth, first, and second order reactions of this data. Thus, a zeroth order concentration would have a y-axis of the concentration versus an x-axis time, a first order reaction would have a y-axis of the natural log of the concentration versus an x-axis of time, and the second order reaction would have a y-axis of (1/concentration) as a function of time. By plotting these relationships and determining which relationship is linear, you can determine whether the data is of a zeroth, first, or second order reaction. This data is of a first order reaction because the graph of natural log of concentration versus time shows a linear relationship, meaning that the data adheres to the relationship of a first order reaction.
Q25.29A
What should be the mass ratio of 210Po/238U in an object that is around 2.4X109 years old? The half-life of 238U is 4.47X109 years. [Hint: one 210Po atom is final decay product of one 238U atom]
S25.29A
Determine the decay constant using the equation λ=A/N
\(\mathrm{0.6934.47*109y=1.55*10^{-10}y^{-1}}\)
Determine the ratio of (Np), number of Pa atoms after 2.4X109 y, to No, initial number of Pa atoms ln(Np/No)=−kt
\(\mathrm{-(1.55*10^{-10}y^{-1}(2.4*10^9y)=-0.372}\)
\(\mathrm{e^{-0.372}=0.689}\)
\(\mathrm{N_pN_o=0.689}\)
For every mole of 238U present initially, after 2.9X109y, there are 0.689 moles of 238U and 0.310 moles of 210Po
Computing mass ratio
\(\mathrm{((0.310mol^{210}Po)/(0.689mol^{238}U))*((1mol^{238}U)/(238gU))*((210gPo)/(1mol^{210}Po))}\)
\(\mathrm{=65.1g^{210}Po/166.12^{238}U}\)
\(\mathrm{=0.392g^{210}PO/1g^{238}U}\)
We are using the relationship that ln(2)/(half-life)= λ from the λ=A/N relationship to first determine the rate constant, λ. Thus, the value of the natural log of 2 is .693, and dividing this by the half-life of 238U (4.47X109 years) will give the rate constant of the decay of 238U to 210Po (it should be .693/(4.47*10^9 years), not 6934.47*109y). Since this nuclear decay is a first-order reaction, we can then use the ln(Np/No)=−kt relationship to determine ratio of the number of Pa atoms after 2.4X109 years, to No, the initial number of Pa atoms. The calculation here is correct. We then continue on to compute the mass ratio. Since the number of moles is conserved in this decay, one mole of 238U will decay to 0.689 moles of 238U and (1 - 0.689)= .310 moles of 210Po. The calculation for converting the moles of 210Po and 238U to their respective masses for a mass ratio is correct.
Q21.1.4
Historically, concrete shelters have been used to protect people from nuclear blasts. Comment on the effectiveness of such shelters.
S21.1.4
Concrete shelters can be used to protect people from nuclear blasts because gamma rays are known to penetrate through most barriers, such as paper, skin, metal, but it can be stopped by layers of concrete. Other alpha and beta rays can be stopped by weaker barriers such as paper or metal.
Q24.6.1
Describe crystal field theory in terms of its
- assumptions regarding metal–ligand interactions.
- weaknesses and strengths compared with valence bond theory.
S24.6.1
1. Crystal field theory (CFT) describes the breaking of orbital degeneracy in transition metal complexes due to the presence of ligands. CFT qualitatively describes the strength of the metal-ligand bonds. Based on the strength of the metal-ligand bonds, the energy of the system is altered. This may lead to a change in magnetic properties as well as color.
In Crystal Field Theory, it is assumed that the ions are simple point charges (a simplification). When applied to alkali metal ions containing a symmetric sphere of charge, calculations of bond energies are generally quite successful. The approach taken uses classical potential energy equations that take into account the attractive and repulsive interactions between charged particles (that is, Coulomb's Law interactions).
\(\mathrm{E∝(q_1q_2)/r}\)
with
- E the bond energy between the charges and
- q1 and q2 are the charges of the interacting ions and
- r is the distance separating them.
2. The valence bond theory describes bonding using hybrid orbitals & electron pairs, as an extension of the electron-dot & hybrid orbital methods (Lewis structures) used for simple molecules. And the crystal field theory is an electrostatic approach, used to describe the split in metal d-orbital energies. It provides an approximate description of the electronic energy levels that determine the UV-Vis spectra, but does not describe the bonding.
A point charge is "an electric charge considered to exist at a single point, and thus having neither area nor volume".

