Extra Credit 16
- Page ID
- 83523
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.5A
Determine whether a forward reaction is spontaneous or non-spontaneous:
- Ag2+(aq)+Cr2+(aq)→Ag+(aq)+Cr3+(aq)
- Sn4+(aq)+2I−(aq)→Sn2+(aq)+I2(s)
S19.5A
1. The forward reaction is given:
Ag2+(aq)+Cr2+(aq)→Ag+(aq)+Cr3+(aq)
The standard reduction potentials are given:
Ag2+(aq)+e- →Ag+(aq) E∘=+1.9V
Cr3+(aq)+2e-→Cr2+(aq) E∘=-0.424V
From this, we can deduce that Ag2+(aq) is reduced(cathode), and Cr2+(aq) is oxidized(anode).
The formula for calculating cell potential at standard conditions is:
E∘cell=E∘cathode−E∘anode
We then plug in the values and solve:
E∘cell=(E∘Ag2+/Ag+)−(E∘Cr3+/Cr2+)
E∘cell=1.9V−(−0.424V)
E∘cell=+2.404V
Because +2.404>0V, the forward reaction is spontaneous.
- The forward reaction is given:
Sn4+(aq)+2I−(aq)→Sn2+(aq)+I2(s)
The standard reduction potentials are given:
Sn4+(aq)+2e-→Sn2+(aq) E∘=+0.154V
I2(s)+2e-→2I−(aq) E∘=+0.535V
From this, we can deduce that Sn4+(aq) is reduced(cathode), and 2I−(aq) is oxidized(anode).
The formula for calculating cell potential at standard conditions is:
E∘cell=E∘cathode−E∘anode
We then plug in the values and solve:
E∘cell=(E∘Sn4+/Sn2+)−(E∘I2/2I-)
E∘cell=(+0.154V)−(+0.535V)
E∘cell=−0.381V
Because -0.381V<0V, the forward reaction is non-spontaneous.
Review
Solution is correct.
1. Ag2+(aq)+Cr2+(aq)→Ag+(aq)+Cr3+(aq) E∘cell=+2.404V spontaneous
2. Sn4+(aq)+2I−(aq)→Sn2+(aq)+I2(s) E∘cell=−0.381V nonspontaneous
Q19.45A
Solve for Ecell of the following voltaic cell
Cu(s)|Cu2+(0.01M)||Cu2+(0.1M)|Cu(s)
S19.45A
Firstly, we must solve for E∘cell with the given SRP and input into the cell potential equation:
E∘cell=E∘cathode−E∘anode
Oxidation(anode): Cu(s)→Cu2++2e− E∘=−0.340V
Reduction(cathode): Cu2+(aq)+2e−→Cu(s) E∘=+0.340V
Thus,
E∘cell=(0.340)−(0.340)
E∘cell=0V
We can now use the Nernst Equation to solve for Ecell:
Ecell=E∘cell−0.02572lnQ
Because E∘cell=0V, we simplify the equation and plug in the given molarities:
Ecell=−0.02572lnQ
=0.02572ln(0.01M/0.1M)
=0.0296V
Review:
Assuming the equation happens at 298K the solution is incorrect.
Using the Nernst Equation
E=E∘cell−0.0592V/n x log Q
Since E∘cell =0V then the equation becomes E=−0.0592V/n x log Q.
n= 2 mole e-
Log Q= log(.01/.1)
-.0592V/2 x log(.01/.1)=.0682V not .0296V
Q21.3A
Sketch the geometric isomers of [CoBr2(en)(CO)2]−[CoBr2(en)(CO)2]−.
S21.3A
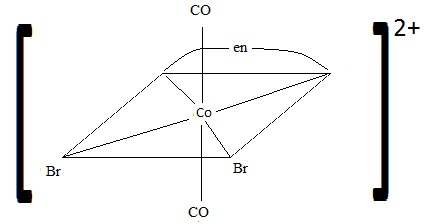
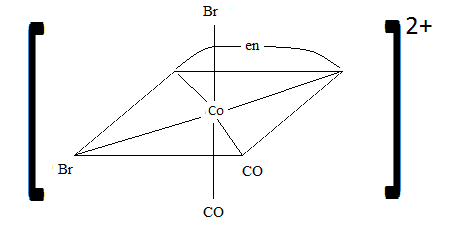
Review
While both of these pictures are correct another possible Geometric isomer is CO and CO both in the bottom like Br and Br in the first picture and then Br and Br in the vertical line. I was not able to sculpt these geometric isomers I could think of but just thinking about the problem this is another geometric isomer.
Q24.5A
In the reaction A→products, 4.50 minutes after the reaction is started, [A]=0.587M. The rate of reaction at this point is rate=−Δ[A]/Δt=2.1×10−2M min−1. Assume that this rate remains constant for a short period of time.
- What is [A] 6.00 minutes after the reaction is started?
- At what time after the reaction is started with [A]=0.56M?
S24.5A
The rate of disappearance of reactants is given:
rate=−Δ[A]/Δt
rate=([A]f-[A]i)/(tf-ti)
1. Plugging in the given values into the equation we can find the molarity after 6 minutes since the reaction started:
2.1×10−2M min−1=-([A]f-0.587)/(6-4.5)
[A]f=0.555M
2. We can also calculate at what time the reaction is at when the molarity is 0.56M.
2.1×10−2M min−1=-(0.56-0.587)/(tf-4.5)
tf=5.786 minutes
Review
This solution is correct and a nicer way to put it is
1. Concentration of Af is .555 M at 6 minutes
2. The Time when [A] is .56 M is tf=5.786 minutes
Q24.47C
For the reversible reaction A+B↔A+B the enthalpy change of the forward reaction is +20kj/mol. The activation energy of the forward reaction is 84 kj/mol.
- What is the activation energy of the reverse reaction?
- Sketch the reaction plot for this reaction
S24.47C
1. To find the activation energy of the reverse reaction, we are given the equation:
Eareverse=ΔH+Eaforward
We then plug in the appropriate values to find the activation energy of the reverse reaction.
Eareverse=20+84
Eareverse=104 kj/mol
2. reaction plot for this reaction:

Review
This solution is correct but the sketch is wrong. Since Dealt H is positive this means there is a gain in energy from the reactants to the products so thats why Delta H is positive, if the reaction plot were exothermic like the picture Delta H would be negative since it is not the reaction plot is endothermic and should look like this. Based on these pictures her graph shows a negative delta H when it is positive so it should be a endothermic graph.


Q25.25C
Suppose a sample with \(\mathrm{\ce{^{170}_{69}Tm}\: }\) has an activity 100 times the detectable limit. How long until the sample's radioactivity is no longer detectable?
S25.25C
The half-life is given:
\(\mathrm{\ce{^{170}_{69}Tm}\: half\: life = 128.6\: days}\)
Given half-life, we are able to determine the rate constant of the reaction:
\(\mathrm{\lambda = \dfrac{0.693}{t_{1/2}}=\dfrac{0.693}{128.6\,d}=0.00539\,d^{-1}}\)
Because we want to know when the sample's radioactivity is no longer detectable, when it originally had activity 100 times the detectable limit, we can then use the integrated rate law:
\(\mathrm{\ln\dfrac{1}{100} = -0.00539\,d^{-1}(t)}\)
\(\mathrm{t=854\: days}\)
Review
This solution is correct and a different way to put the answer is The Radioactivity of 170Tm is no longer detectable after 854 days or 2 years 4 months and 4 days.
Q18.3
Classify each of the following substances as an oxidizing agent, reducing agent or both. List the oxidizing agents in order of decreasing strength; list the reducing agents in order of decreasing strength (use SRP Table):
Ni(s), H+(aq), Au(s), Cl2(g), Sn2+(aq), Mg(s), Fe2+(aq)
S18.3
The standard reduction potentials are taken from the SRP Table:
Mg2+(aq) + 2e- ⇌ Mg(s) E∘=-2.356
Ni2+(aq) + 2e- ⇌ Ni(s) E∘=–0.257
Sn2+(aq) + 2e- ⇌ Sn(s) E∘=-0.14
2H+(aq)+2e-⇌ H2(g) E∘=0V
Fe3+(aq) + e- ⇌ Fe2+(aq) E∘=0.771
Cl2(g) + 2e- ⇌ 2Cl-(aq) E∘=1.396
Au3+ + 3e− ⇌ Au(s) E∘=1.52
From the above standards we can conclude:
Mg(s) is a reducing agent; it has a negative reduction potential
Ni(s) is a reducing agent; it has a negative reduction potential
Sn2+(aq) is a reducing agent; it has a negative reduction potential
H+(aq) is both an oxidizing and reducing agent; it reduction potential is neither negative nor positive
Fe2+(aq) is an oxidizing agent; it has a positive reduction potential
Cl2(g) is an oxidizing agent; it has a positive reduction potential
Au(s) is an oxidizing agent; it has a positive reduction potential
And we can now order the substances in order of decreasing strength:
reducing agents: Mg(s)>Ni(s)>Sn2+(aq)
oxidizing agents: Au(s)>Cl2(g)>Fe2+(aq)
both reducing and oxidizing agent: H+(aq)
Review
This answer is correct and another way although this answer is thorough is just
Hydrogen is both a reducing and oxidizing agent.
Au(s)>Cl2(g)>Fe2+(aq) For Oxidizing good at taking electrons
Mg(s)>Ni(s)>Sn2+(aq) For Reducing good at giving electrons
Q21.3.9
When a star reaches middle age, helium-4 is converted to short-lived beryllium-8 (mass = 8.00530510 amu), which reacts with another helium-4 to produce carbon-12. How much energy is released in each reaction (in megaelectronvolts)? How many atoms of helium must be “burned” in this process to produce the same amount of energy obtained from the fusion of 1 mol of hydrogen atoms to give deuterium?
S21.3.9
Information we will need to calculate how much energy is released:
E=Δmc2
speed of light: 3.00×108 m/s
1 amu = 1.66×10−27 Kg = 1.4924×10−10 J
1.6×10−13 J = 1 MeV
mass of a free proton: 1.007825 amu
mass a free neutron: 1.008665 amu
The first reaction is:
2\(\mathrm{\ce{^{4}_{2}He}\:}\)→ \(\mathrm{\ce{^{8}_{4}Be}\:}\)
In order to calculate how much energy is released, we must first calculate the change in mass:
total mass before the reaction: 2(4.002602 amu)=8.005205 amu
total mass after the reaction: 8.00530510 amu
change in mass: (8.00530510)-(8.005205)=1.011×10−4 amu
We can now calculate the nuclear binding energy:
E=(1.011×10−4 amu)((1.4924×10−10 J)/(1 amu))((1 MeV)/(1.6×10−13 J))(3.00×108 m/s)2
E= 8.487×1015 MeV
The second reaction is:
\(\mathrm{\ce{^{8}_{4}Be}\:}\) +\(\mathrm{\ce{^{4}_{2}He}\:}\)→ \(\mathrm{\ce{^{12}_{7}C}\:}\)+ß-
In order to calculate how much energy is released, we must first calculate the change in mass:
total mass before the reaction:(8.00530510)+(4.002602)=12.0079071 amu
total mass after the reaction: 12.0107 amu
change in mass: (12.0107)-(12.0079071)=0.0027929 amu
We can now calculate the nuclear binding energy:
E=(0.0027929 amu)((1.4924×10−10 J)/(1 amu))((1 MeV)/(1.6×10−13 J))(3.00×108 m/s)2
E=2.345×1017 MeV
The Review
The Solution covers the first part of the question and is correct
Energy released in 1st step:E= 8.487×1015 MeV
Energy released in 2nd Step:E=2.345×1017 MeV
Addition is 2.43x1017
But does not cover the second part of the question
mass of H before reaction 1.00794 amu
Mass of Deuterium after reaction 2.01410178 amu
E=Δmc2 Δm=2.01410178-1.00794 amu=1.00616178amu
E= (1.00616178 amu)(1.4924×10−10 J)/(1 amu))((1 MeV)/(1.6×10−13 J))(3.00×108 m/s)2
E=8.45x1019MeV
8.45x1019MeV /2.43x1017 MeV= 348 moles of Helium using the whole process of Helium to Carbon.

