Extra Credit 33
- Page ID
- 82792
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.5.1
What are the desirable qualities of an electric battery?
S17.5.1
The purpose of a battery is to store potential energy in its chemical form until it is needed where it is then converted to electric energy. Desirable qualities of an electric battery include low cost of materials, low toxicity and ease of disposal, and a high capacity. Furthermore, they should be lightweight, resistant to heat and humidity, and avoid leaks when the battery is used according to instruction.
Q12.1.6
Consider the following reaction in aqueous solution:
\(5Br^− (aq) + BrO^{3-} (aq) + 6H^+ (aq) \rightarrow 3Br_2 (aq) + 3H_2O (l)\)
If the rate of disappearance of Br–(aq) at a particular moment during the reaction is 3.5 × 10−4 M s−1, what is the rate of appearance of Br2(aq) at that moment?
S12.1.6
1. Define the reaction rate of the reaction.
NOTE: The reaction rate for a reaction is the measure of the change in the concentration of the reactants per unit of time. It can also be the measure of the change in concentration of the products per unit of time.
\(rate=\frac{\Delta concentration}{\Delta time}\)
Therefore, for the reaction \(A + B \rightarrow C\), the reaction rate would be
\(rate = \frac{- \Delta A}{\Delta t} = \frac{- \Delta B}{\Delta t} = \frac{\Delta C}{\Delta t}\)
So in our case, the reaction rate for \(5Br^− (aq) + BrO^{3-} (aq) + 6H^+ (aq) \rightarrow 3Br_2 (aq) + 3H_2O (l)\), would be
\(rate = \frac{- \Delta[Br^-]}{5\Delta t} = \frac{- \Delta[BrO^-3]}{\Delta t} = \frac{- \Delta[H^+]}{6\Delta t} = \frac{\Delta[Br_2]}{3\Delta t} = \frac{\Delta[H_2O]}{3\Delta t}\)
2. Since we are trying to find the rate of appearance of \(Br_2\) (aq) at the moment when the rate of disappearance of \(Br^-\) (aq) is \(3.5 \times 10^{-4} \frac{M}{s}\), we use their rates and set them equal to each other.
\(rate = \frac{- \Delta[Br^-]}{5\Delta t} = \frac{\Delta[Br_2]}{3\Delta t}\)
3. Plug in the rate of disappearance of \(Br^-\) (aq) into the rate equation, then solve for the rate of appearance of \(Br_2\) (aq).
\(rate = \frac{3.5 \times 10^{-4}}{5} = \frac{\Delta[Br_2]}{3\Delta t}\)
\(\frac{\Delta[Br_2]}{\Delta t} = 3 \times \frac{3.5 \times 10^{-4}}{5}\)
\(\frac{\Delta[Br_2]}{\Delta t} = 2.1 \times 10^{-4} \frac{M}{s}\)
Q12.5.5
Describe how graphical methods can be used to determine the activation energy of a reaction from a series of data that includes the rate of reaction at varying temperatures.
S12.5.5
This method is based on the Arrhenius equation which can be used to show the effect of a change of temperature on the rate constant, and therefore on the rate of reaction.
The rate constant is different from reaction rat in that the reaction rate is the measure of how fast or slow a chemical reaction takes place while a rate constant is a constant that shows the relationship between the reaction rate and the concentrations of the reactants or products.
For example, for the reaction \(A + B \rightarrow C\), the rate law would be:
\(rate = k[A]^a[B]^b\)
k = rate constant
[A] = concentration of reactant A
a = order of reaction with respect to A
[B] = concentration of reactant B
b = order of reaction with respect to B
However, the rate constant remains constant only if you are changing the concentration of the reactants. If you change the temperature or the catalyst of the reaction, the rate constant will change and this is demonstrated by the Arrhenius equation:
\(k = Ae^\frac{-E_a}{RT}\)
\(ln \left(\frac{k_1}{k_2}\right) = \left(\frac{-E_a}{R}\right)\left(\frac{1}{T_1} - \frac{1}{T_2}\right)\)
k = rate constant
A = frequency factor
\(E_a\) = activation energy
e = exponential function, \(e^x\)
R = gas constant
T = temperature (K)

In other words, the activation energy of a reaction, \(E_a\), from a series of data that includes the rate of reaction, k, at varying temperatures can be determined by graphing it on a plot of \(\ln k\) versus \(\frac{1}{T}\). You can then use the slope of the graph you have plotted to solve for \(E_a\) by setting the slope equal to \(\frac{-E_a}{R}\).
Q21.3.8
For the reaction \(^{14}_6C \rightarrow ^{14}_7N + \,?\), if 100.0 g of carbon reacts, what volume of nitrogen gas \(N_2\) is produced at 273 K and 1 atm?
S21.3.8
Recall the ideal gas equation, \(PV = nRT\) in standard temperature and pressure (STP), in which
P = pressure (1 atm)
V = volume (L)
n = number of moles (mol)
R = gas constant, \(0.008206 \frac{L \cdot atm}{mol \cdot K}\) or \(8.3145 \frac{J}{mol \cdot K}\)
T = temperature (273 K)
1. Using stoichiometry, convert the 100 grams of carbon into moles of \(N_2\) (molar mass of carbon is ~12g/mol).
\((\frac{100 g C}{}) \times (\frac{1 mol C}{12 g C}) \times (\frac {1 mol N_2}{1 mol C}) = 8.33 mol N_2\)
2. Plug the given values into the ideal gas equation in order to find the volume of \(N_2\).
\(PV = nRT\)
\((1 atm)(V) = (8.33mol N_2)(0.008206 \frac{L \cdot atm}{mol \cdot K})(273K)\)
\(V = 18.66 L\,of \,N_2\,gas\)
Q20.2.4
Single-displacement reactions are a subset of redox reactions. In this subset, what is oxidized and what is reduced? Give an example of a redox reaction that is not a single-displacement reaction.
S20.2.4
A redox reaction, or oxidation-reduction reaction, is a chemical reaction that involves the transferring of electrons between species as one is being oxidized, the reducing agent, and the other reduced, the oxidizing agent. In order to identify which of the species is the reducing or oxidizing agent, you must look at their oxidation numbers.
Oxidation or the one known as the reducing agent is the loss of electrons. Reduction or the one known as the oxidizing agent is the gaining of electrons.
A helpful mnemonic to remember this would be OIL RIG as Oxidation Is Loss Reduction Is Gain.
In single-displacement reactions, it involves “replacing” an element in the reactant with an element in the product. For example, in \(A + BC \rightarrow AB + C\), C in the BC compund on the reactant side is being replaced by A to produce the AB compound.
Another example of this would be
\(Cl_2 + 2KBr \rightarrow Br_2+ 2KCl\)
as Br in KBr is being replaced by \(Cl_2\), with K acting as a spectator ion. The half-reactions in this for this reaction are:
\(Cl_2+2e^- \rightarrow 2Cl^-\)
\(2Br^- \rightarrow Br_2+ 2e^-\)
In this, \(Cl_2\) would be reduced from an oxidation number of (0) to (-1) while Br in KBr is oxidized from an oxidation number of (-1) to (0).
Redox reactions do not only have single-displacement reactions but also other types of redox reactions such as combination, combustion, decomposition, and disproportionation reactions.
An example of a redox reaction that is not a single-displacement reaction would be
\(CH_4 + 2O_2 \rightarrow 2H_2O + CO_2\)
which is a combustion reaction, another type of redox reaction. The balanced half-reactions are:
\(2O_2+ 8e^- \rightarrow 4O^{2-}\)
\(C^{-4} \rightarrow C^{+4}+ 8e^-\)
\(O_2\) has been reduced from (0) to (-2), while C has been oxidized from (-4) to (+4).
Q20.4.23
If you place Zn-coated (galvanized) tacks in a glass and add an aqueous solution of iodine, the brown color of the iodine solution fades to a pale yellow. What has happened? Write the two half-reactions and the overall balanced chemical equation for this reaction. What is \(E_\left(cell\right)^°\)?
S20.4.23
When adding an aqueous iodine solution to the zinc-coated tacks in a glass, the brown color of the iodine solution turns to a pale yellow because the iodine is reduced due to the presence of zinc. Zinc holds onto its electrons very weakly and as a result, when iodine is added, it releases them to form zinc ions and iodide ions.
\(Zn(s) \rightarrow Zn^{2+}(aq) + 2e^-\)
\(I_2(aq) + 2e^- \rightarrow 2I^-(aq)\)
Overall reaction: \(Zn(s) + I_2(aq) \rightarrow Zn^{2+}(aq) + 2I^-(aq)\)
\(I_2\) is reduced while \(Zn\) is oxidized
1. To determine \(E_\left(cell\right)^°\), look up the standard reduction potentials (SRP) for the reduction half-reaction and oxidation half-reaction.
\(E_\left(reduction of I_2\right) ^° = +0.54\)
\(E_\left(reduction of Zn^{2+}\right) ^° = -0.76\)
As you can see in the SRP table, even though we are given the equation for the reduction of Zn, \(Zn^{2+} + 2e^- \rightarrow Zn\), the equation for \(E_\left(cell\right) ^°\), \(E_\left(cell\right)^° = E_\left(cathode\right)^°- E_\left(anode\right)^°\), accounts for the oxidation potential with the "-" sign. You can therefore plug these values into the equation directly without changing the sign of any numbers.
2. Plug in the numbers into the equation \(E_\left(cell\right)^° = E_\left(cathode\right)^°- E_\left(anode\right)^°\) to find \(E_\left(cell\right)^°\), where the \(E_\left(cell\right)^°\) value for the reduction of \(I_2\) corresponds to \(E_\left(cathode\right)^°\) and the \(E_\left(cell\right)^°\) for \(Zn\) corresponds to \(E_\left(anode\right)^°\).
\(E_\left(cell\right)^°= 0.54 - (-0.76) = 1.30V\)
\(E_\left(cell\right)^°\) is 1.30V.
Q20.9.8
What mass of copper metal is deposited if a 5.12 A current is passed through a \(Cu(NO_3)_2\) solution for 1.5 h.
S20.9.8
1. Write out the chemical equation you will need for this problem. In this case, since we are working with copper, the equation will be
\(Cu^{2+} + 2e^- \rightarrow Cu\)
2. We now know that for every 2 moles of electrons transferred, we get 1 mole of copper. We now need to plug our given values into the following equation:
\[n_e = \frac{I×t}{F}\]
Where ne is the total number of moles of electrons transferred, I is the current in amps, t is the time in seconds and F is Faraday's constant (96485 coulombs/mol of electrons)
\( (5.12 A) \times (1.5hrs) \times \left(\frac{3600s}{1hr}\right) \times \left(\frac{1 mol\,e^-}{96485 C}\right) = 0.2866 mol\)
3. Finally, we convert moles of electrons to moles of copper by dividing by 2 according to our half equation above, then convert moles of copper to grams of copper using stoiciometry:
\((0.2866 mol) \times \left(\frac{1 mol Cu(NO_3)_2}{2 mol\,e^-}\right) \times \left(\frac{1molCu}{1molCu(NO_3)_2}\right) \times \left(\frac{63.546gCu}{1 mol Cu}\right) = 9.105g\,of \,Cu\)
Q14.6.6
Cyclopropane, a mild anesthetic, rearranges to propylene via a collision that produces and destroys an energized species. The important steps in this rearrangement are as follows:
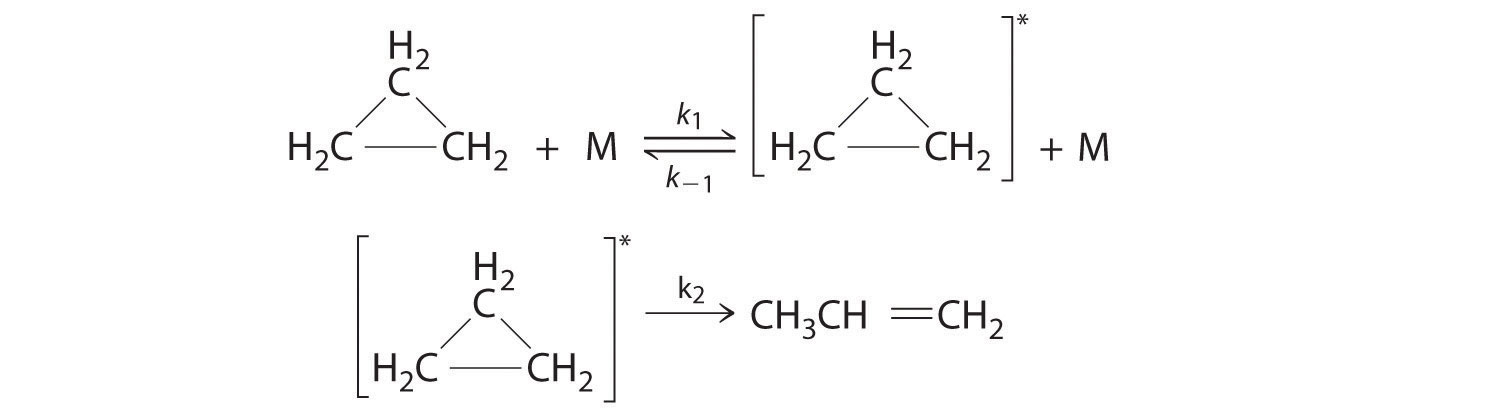
where M is any molecule, including cyclopropane. Only those cyclopropane molecules with sufficient energy (denoted with an asterisk) can rearrange to propylene. Which step determines the rate constant of the overall reaction?
S14.6.6
According to chemical kinetics, in order to identify the rate constant of the overall reaction you must find the rate limiting step, the slowest step in the reaction mechanism, as the overall reaction cannot proceed faster than the slowest step.
Since the first step, \(k_1\) and \(k_{-1}\) is an equilibrium equation where a specific amount of energy is not specified and equilibrium equations reach equilibrium quickly, the second step, \(k_2\), must be the rate limiting step. Furthermore, since cyclopropane molecules need a sufficient amount of energy in order to rearrange into propylene, the reaction would likely proceed slowly as we would need to wait until sufficient energy is accumulated, which may take some time, until the molecule can rearrange and the reaction can complete. With \(k_2\) being the rate limiting step, that means the second step is the slowest and determines the rate constant of the overall reaction.

