Claisen Rearrangement
- Page ID
- 66872
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisenin 1912. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.
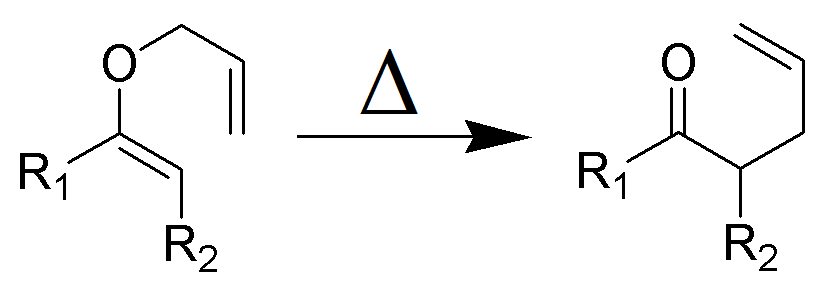
Figure 1: Wikimedia (Own work) [GFDL CC-BY-SA-3.0; ~K)
The Claisen rearrangement is the first recorded example of a [3,3]-sigmatropic rearrangement.
The Claisen rearrangement is an exothermic, concerted (bond cleavage and recombination) pericyclic reaction. Woodward–Hoffmann rules show a suprafacial, stereospecific reaction pathway. The kinetics are of the first order and the whole transformation proceeds through a highly ordered cyclic transition state and is intramolecular. Crossover experiments eliminate the possibility of the rearrangement occurring via an intermolecular reaction mechanism and are consistent with an intramolecular process.
There are substantial solvent effects observed in the Claisen rearrangement, where polar solvents tend to accelerate the reaction to a greater extent. Hydrogen-bonding solvents gave the highest rate constants. For example, ethanol/water solvent mixtures give rate constants 10-fold higher than sulfolane. Trivalent organoaluminium reagents, such as trimethylaluminium, have been shown to accelerate this reaction.
The first reported Claisen rearrangement is the [3,3]-sigmatropic rearrangement of an allylphenyl ether to intermediate 1, which quickly tautomerizes to an ortho-substituted phenol.
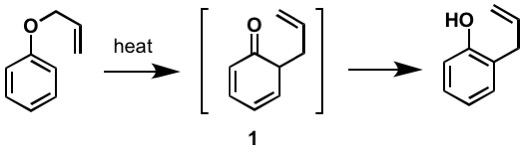
Figure 2: By Kchemyoung (Own work) [CC BY-SA 4.0, Kchemyoung)
Meta-substitution affects the regioselectivity of this rearrangement. For example, electron withdrawing groups (e.g. bromide) at the meta-position direct the rearrangement to the ortho-position (71% ortho-product), while electron donating groups (e.g. methoxy), direct rearrangement to the para-position (69% para-product). Additionally, presence of ortho-substituents exclusively leads to para-substituted rearrangement products (tandem Claisen and Cope rearrangement).
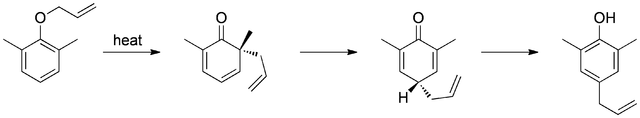
Figure 3: (Public Domain; RAN 10).
If an aldehyde or carboxylic acid occupies the ortho or para positions, the allyl side-chain displaces the group, releasing it as carbon monoxide or carbon dioxide, respectively.
Bellus–Claisen rearrangement
The Bellus–Claisen rearrangement is the reaction of allylic ethers, amines, and thioethers with ketenes to give γ,δ-unsaturated esters, amides, and thioesters. This transformation was serendipitously observed by Bellus in 1979 through their synthesis of a key intermediate of an insecticide, pyrethroid. Halogen substituted ketenes (R1, R2) are often used in this reaction for their high electrophilicity. Numerous reductive methods for the removal of the resulting α-haloesters, amides and thioesters have been developed. The Bellus-Claisen offers synthetic chemists a unique opportunity for ring expansion strategies.
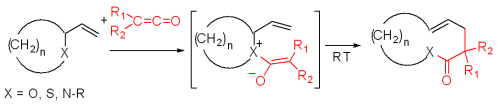
at the English language Wikipedia [CC-BY-SA-3.0 , GFDL, CC-BY-SA-3.0 or CC BY-SA 3.0 , from Wikimedia Commons
Eschenmoser–Claisen rearrangement
The Eschenmoser–Claisen rearrangement proceeds by heating allylic alcohols in the presence of N,N-dimethylacetamide dimethyl acetal to form γ,δ-unsaturated amide. This was developed by Albert Eschenmoser in 1964.Eschenmoser-Claisen rearrangement was used as a key step in the total synthesis of morphine.
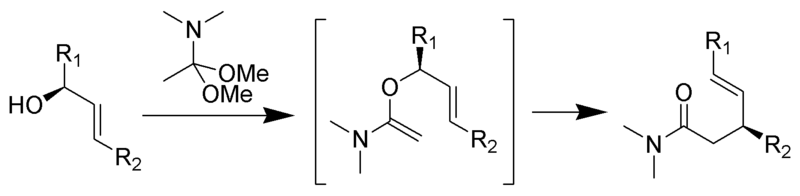
Wikimedia CommonsBy ~K (Own work) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons
Ireland–Claisen rearrangement
The Ireland–Claisen rearrangement is the reaction of an allylic carboxylate with a strong base (such as lithium diisopropylamide) to give a γ,δ-unsaturated carboxylic acid. The rearrangement proceeds via silylketene acetal, which is formed by trapping the lithium enolate with chlorotrimethylsilane. Like the Bellus-Claisen (above), Ireland-Claisen rearrangement can take place at room temperature and above. The E- and Z-configured silylketene acetals lead to anti and syn rearranged products, respectively.[30] There are numerous examples of enantioselective Ireland-Claisen rearrangements found in literature to include chiral boron reagents and the use of chiral auxiliaries.
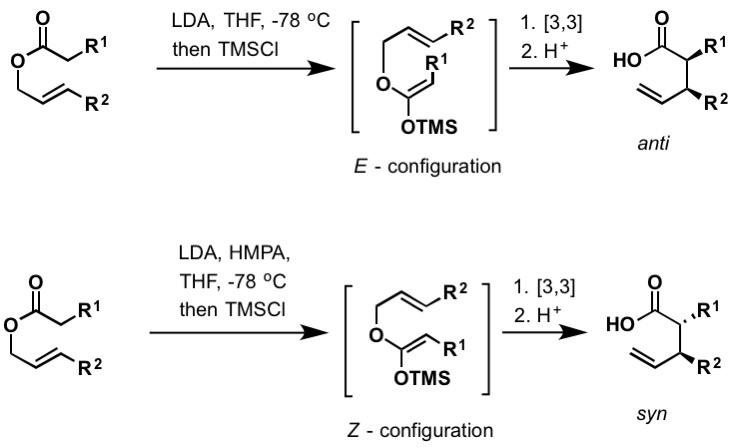
Figure 5: (CC BY-SA 4.0; Kchemyoung)
Johnson–Claisen rearrangement
The Johnson–Claisen rearrangement is the reaction of an allylic alcohol with an orthoester to yield a γ,δ-unsaturated ester. Weak acids, such as propionic acid, have been used to catalyze this reaction. This rearrangement often requires high temperatures (100 to 200 °C) and can take anywhere from 10 to 120 hours to complete. However, microwave assisted heating in the presence of KSF-clay or propionic acid have demonstrated dramatic increases in reaction rate and yields.
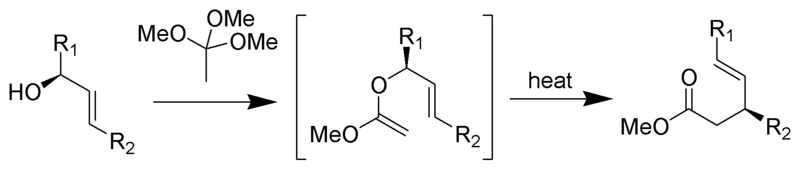
Mechanism:
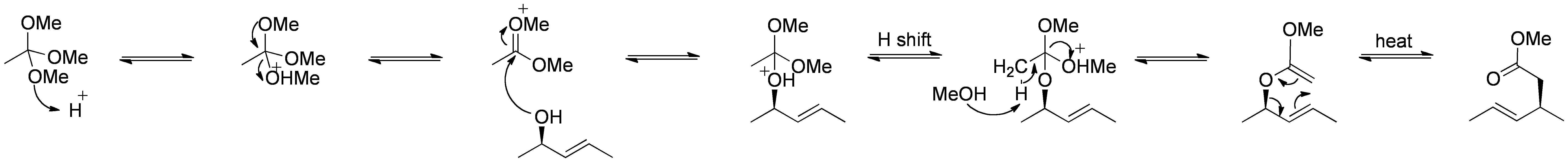
By Self Made by RAN 10 (-) [CC0], via Wikimedia Commons
Photo-Claisen rearrangement
The photo-Claisen rearrangement is closely related to the photo-Fries rearrangement, that proceeds through a similar radical mechanism. Aryl ethers undergo the photo-Claisen rearrangement, while the photo-Fries rearrangement utilizes aryl esters.
Hetero-Claisens
Aza–Claisen
An iminium can serve as one of the pi-bonded moieties in the rearrangement.
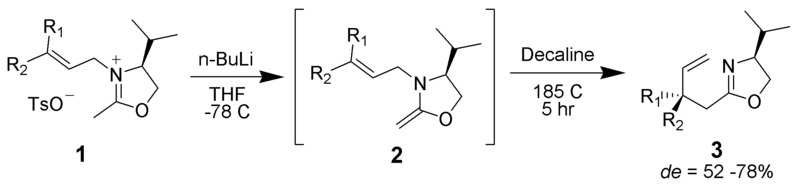
By No machine-readable author provided. ~K assumed (based on copyright claims). [Public domain], via Wikimedia Commons
Chromium oxidation
Chromium can oxidize allylic alcohols to alpha-beta unsaturated ketones on the opposite side of the unsaturated bond from the alcohol. This is via a concerted hetero-Claisen reaction, although there are mechanistic differences since the chromium atom has access to d- shell orbitals which allow the reaction under a less constrained set of geometries.
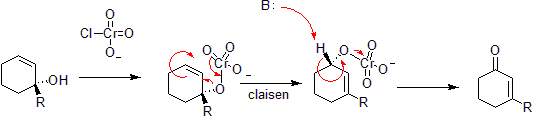
By The original uploader was Takometer at English Wikipedia [CC BY 2.5, via Wikimedia Commons
Chen–Mapp reaction
The Chen–Mapp reaction also known as the [3,3]-Phosphorimidate Rearrangement or Staudinger–Claisen Reaction installs a phosphite in the place of an alcohol and takes advantage of the Staudinger reduction to convert this to an imine. The subsequent Claisen is driven by the fact that a P=O double bond is more energetically favorable than a P=N double bond.
.png?revision=1&size=bestfit&width=660&height=112)
By Howcheng at en.Wikipedia [CC BY 2.5], via Wikimedia Commons
Overman rearrangement
The Overman rearrangement (named after Larry Overman) is a Claisen rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides.
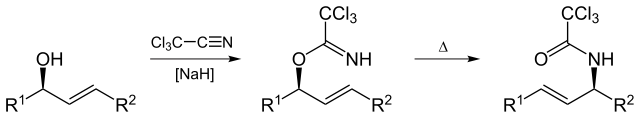
By Yikrazuul (Own work) [Public domain], via Wikimedia Commons
Overman rearrangement is applicable to synthesis of vicinol diamino comp from 1,2 vicinal allylic diol.
Zwitterionic Claisen rearrangement
Unlike typical Claisen rearrangements which require heating, zwitterionic Claisen rearrangements take place at or below room temperature. The acyl ammonium ions are highly selective for Z-enolates under mild conditions
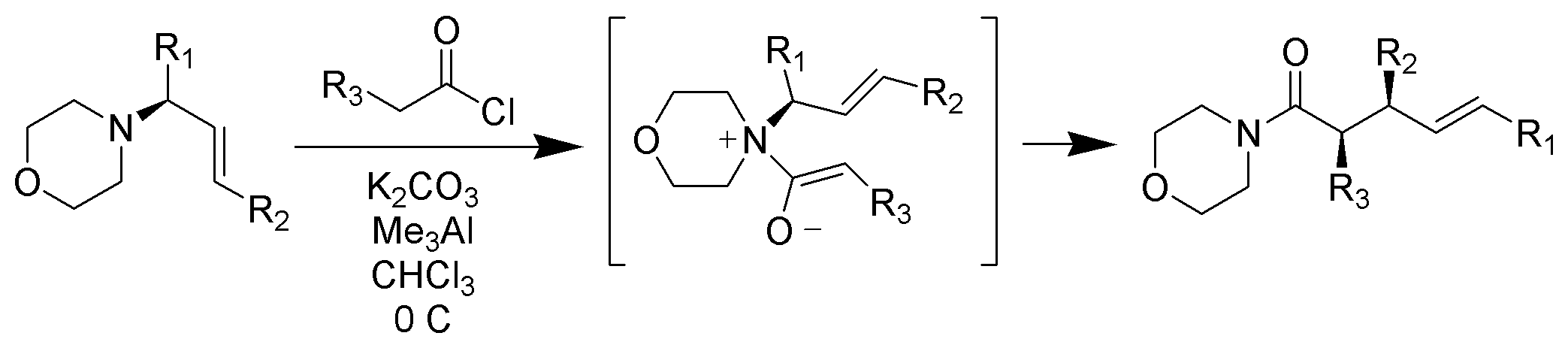
Claisen rearrangement in nature
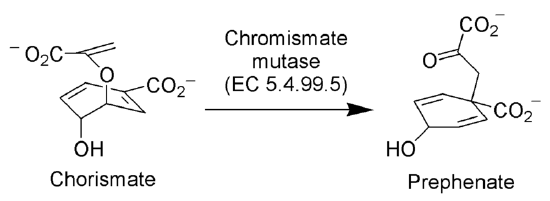
The enzyme Chorismate mutase (EC 5.4.99.5) catalyzes the Claisen rearrangement of chorismate ion to prephenate ion, a key intermediate in the shikimic acid pathway (the biosynthetic pathway towards the synthesis of phenylalanine and tyrosine).
By No machine-readable author provided. ~K assumed (based on copyright claims). [Public domain], via Wikimedia Commons