Table Basics
- Page ID
- 620
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
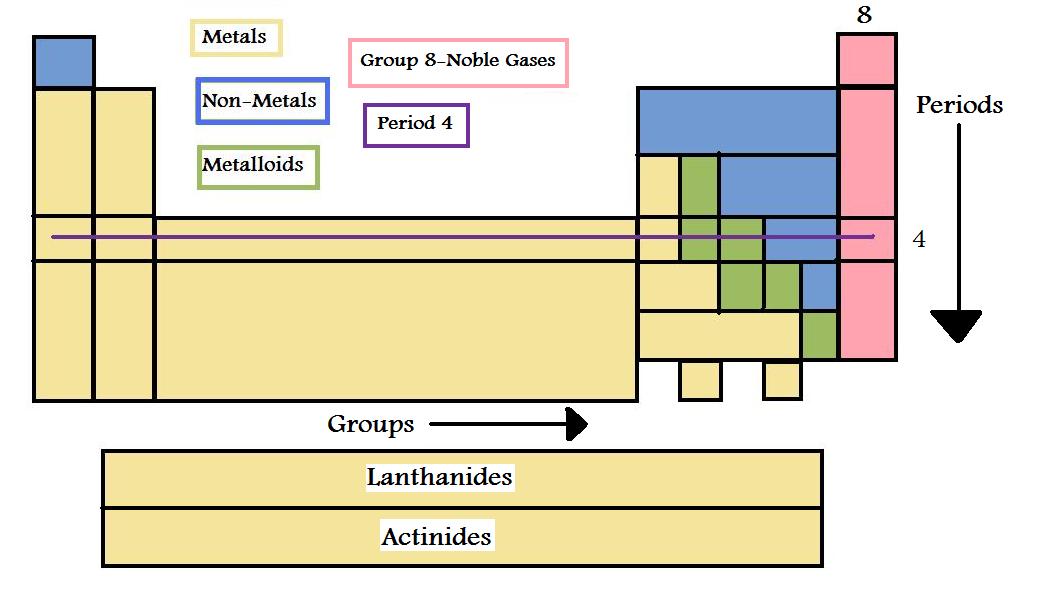
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Periodic table characterizes the known elements in increasing order of atomic number. It starts on the top right hand corner with Hydrogen and continue from left to right which then repeats in the horizontal row below the last element. This is not just a list of elements it is organized in very different ways like properties and atomic mass. At first glance the periodic table may seem disorganized with only a couple elements on the top row and a block on the last row but it is very specific in the way that they are organized.
The Periodic Table is organized by Properties
Elements themselves can be one of the following metals, nonmetals and metalloids.
Metals are:
- Solid at room temperature (except for mercury which will be liquid)
- Malleable (the ability to be flattened into a sheet)
- Ductile (the ability to be made into wire wires)
- good conductors of heat and electricity
- shiny in appearance
Non-metals are the complete opposite so they are:
- poor conductors of heat
- At different phases at room temperature
- Nitrogen, Oxygen, and Chloride are gases
- Silicon and Sulfur are brittle solids
- Bromine is liquid
Metalloids have the properties from both metals and non-metals.
Position
One way that the elements are organized is vertically in groups or families. An example of this is group 8, the noble gases with include Helium, Neon and Argon which has a specific name called alkali metals. Group 1 isn’t the only family with a special name there is group 17 which includes Fluorine and Chlorine, that is called the halogens. When looking at groups, elements at the top are the beginning of the group and the ones at the bottom are the end of the group. The main group elements are the ones in groups 1, 2 and the ones ranging from 13 – 18 and the metals in between 3 and 12 are the transition metals.
Elements are organized in horizontally in periods. There are a total of 7 groups in the periodic table and each vary in how many elements they contain. Period 1 only has two elements, Hydrogen and Helium. Period 2 has eight elements whereas group 6 has the most with the addition of the top section of the block of elements on the bottom of the table called lanthanides. Below them are the actinides which follow the same rules as the lanthanide but in group 7.
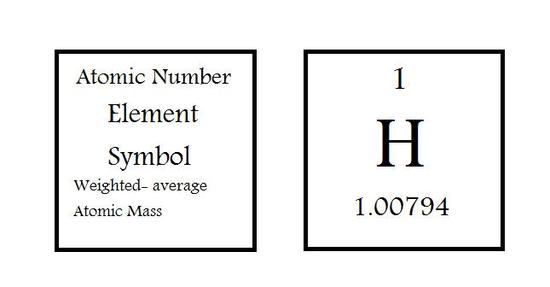
The individual elements are represented in their own blocks by stating the atomic number (Z) on the top of the box, in the middle is the elements symbol and on the bottom is the average atomic mass of the element. This information can differ in the type of periodic table you are looking at. Some can include information about the actual chemical name or omit information described above.
Practice problems
Determine whether the following elements are metals, non-metals or metalloids,
- calcium,
- phosphorus,
- silicon
- krypton
Identify the group and period that the following elements are in:
- hydrogen,
- aluminum
- silver
Classify which elements are considered as the main group or transition metals. If they are transition metals, state if they are lanthanides or actinides. The elements are:
- Magnesium,
- Lanthanide
- Uranium
- Holmium
- Selenium
Arrange the elements from the lowest to highest group number: nitrogen, fluorine, boron, oxygen and carbon.
Arrange the following elements from the lowest to highest period number: aluminum, polonium, germanium, and antimony.
From looking at the periodic table, information about the following elements:
- cobalt,
- barium
- chromium.
Answers
- Determine whether the following elements are metals, non-metals or metalloids:
- Calcium is a metal,
- phosphorus is a non-metal
- silicon is a metalloid
- krypton is a nonmetal
- Identify the group and period that the following elements are in:
- Hydrogen is in group 1and period 1.
- Aluminum is in the group 13 but group 3 for the main group elements, the period is 3.
- Silver is Ag which is in group 11 and period 5.
- Identify which elements are considered as the main group or transition metals. If they are transition metals, state if they are lanthanides or actinides. The elements are:
- Magnesium, Selenium, and Lanthanide are all main group elements.
- Uranium is a transition metal which is part of the Actinide series.
- Holmium is a transition metal as well but is part of the Lanthanides.
- Arrange the elements from the lowest to highest group number: nitrogen, fluorine, boron, oxygen and carbon.
Boron, carbon, nitrogen, oxygen, fluorine
- Arrange the following elements from the lowest to highest period number: aluminum, polonium, germanium, and antimony.
aluminum, germanium, antimony, and polonium
6. From looking at the periodic table, information about the following elements:
This depends on what period table you use!
- Cobalt has an atomic number (Z) of 27. Elemental symbol of Co and weighs 58.693
- Barium has an atomic number (Z) of 56. Elemental symbol of Ba and weighs 137.327.
- Chromium has an atomic number (Z) of 24. Elemental symbol of Cr and weighs 51.9961.
References
- Petrucci, Ralph, William Harwood, Geoffrey Herring, and Jeffry Madura. General Chemistry. 9th ed. Upper Saddle River, New Jersey: Pearson Prentice Hall, 2007
Contributors and Attributions
- Jayne Aclan (UCD)