23.1: Introduction
- Page ID
- 34540
Main Group Organometallic Chemistry
Introduction
Organometallic compounds have been known and studied for over 250 years. Many of these early compounds were prepared directly from the metal by oxidative addition of alkyl halides. All these metals have strong or moderately negative reduction potentials, with lithium and magnesium being the most reactive. Halide reactivity increases in the order: Cl < Br < I.
In 1757 Louis Claude Cadet de Gassicourt prepared what is believed to be the first synthetic organometallic compound and it was isolated from arseneous oxide (As2O3) and potassium acetate. The mixture was named after him "Cadet's fuming liquid" from which came cacodyl oxide.
4 KCH3COO + As2O3 → As2(CH3)4O + 4 K2CO3 + CO2 → → As2(CH3)4
which disproportionates to produce among other things cacodyl, As2(CH3)4. The poisonous garlic-smelling red oily-liquid is unstable undergoing spontaneous combustion in dry air.
Another organoarsenic compound, Salvarsan, was one of the first pharmaceuticals, and earned a Nobel Prize in Medicine for Paul Ehrlich in 1908 (jointly with Ilya Ilyich Mechnikov). Its activity against syphilis was discovered as a result of the first largescale testing of chemicals and had a code name of 606 since it was apparently the 606th chemical that had been tested in Ehrlich's laboratory in his quest for the "magic bullet". The compound was synthesised by reaction of 3-nitro-4- hydroxyphenylarsonic acid with dithionite.
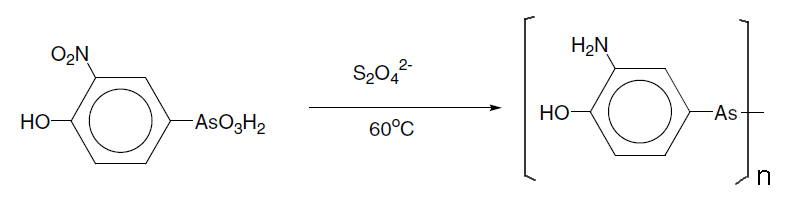
The structure has only recently been characterised as a mixture of polyarsines (AsR)n n= 3-6, and originally it was proposed that it was a dimer with an As=As double bond.
trimer |
pentamer |
Edward Frankland prepared the first organozinc compound (diethylzinc) in 1848 from zinc metal and ethyl iodide, he went on to improve the synthesis of diethylzinc by using diethyl mercury as starting material.
2R-X + 2Zn → R2Zn + ZnX2
Grignard reagents are formed via the action of an alkyl or aryl halide on magnesium metal.
R-X + Mg → R-Mg-X
Victor Grignard was jointly awarded the 1912 Nobel Prize in Chemistry.
Carl Jacob Lowig (1803-1890) reported the preparation of the first alkyltin and alkyllead compounds in 1852/3. He reacted ethyl iodide and Sn/Na or Pb/Na alloy
Wilhelm Johann Schlenk discovered organolithium compounds around 1917.
R-X + 2Li → R-Li + LiX
He also investigated free radicals and carbanions and discovered (together with his son) that organomagnesium halides are capable of participating in a complex chemical equilibrium, now known as a Schlenk equilibrium.
2RMgX → MgX2 + MgR2
Karl Ziegler
His work with free radicals led him to the organo compounds of the alkali metals. He discovered that ether scission opened a new method of preparing sodium and potassium alkyls; later (1930) he directly synthesized lithium alkyls and aryls from metallic lithium and halogenated hydrocarbons. This important discovery made the lithium compounds as readily available as the familiar Grignard reagents.
Ziegler is perhaps best remembered for his work with Giulio Natta on what are called Ziegler-Natta catalysts. These catalysts are typically based on titanium compounds and organometallic aluminium compounds, such triethylaluminium, (C2H5)3Al and are used to polymerize terminal 1-alkenes.
n CH2=CHR → -[CH2-CHR]n-
Together they won the Nobel Prize in Chemistry in 1963.
Classification of organometallic compounds

Examples will be selected from the circled elements.
The organometallic compounds to be considered in this course are those containing a M-C bond, excluding carbonyls (M-CO), cyanides (M-CN) or carbides (M-C). A useful subdivision is by the type of M-C bond:
- ionic - with most Group 1 elements
- covalent - with many Group 12, 13, 14 and 15 elements
- electron deficient - with Li, Be, Mg, B, Al
Ionic
Ionic organometallic compounds are generally formed from elements such as sodium, potassium etc. where the metals are considered electropositive. If the organic groups are able to delocalise the negative charge over several carbon atoms then less electropositive elements like magnesium can also form ionic compounds, eg Cp2Mg. In this case the charge is considered to be delocalised over each of the five carbon atoms in each ring.
Covalent
The simplest model of the M-C bond is where it consists of essentially a single covalent 2-electron bond. These compounds are often volatile and are comparable to typical organic compounds being soluble in organic solvents.
Electron deficient
Electron deficient organometallic compounds are generally associated with elements that have less than half-filled valence shells and are designated as such because of an insufficient number of valence electrons to allow all the atoms to be linked by traditional two-electron two-centre bonds. The compounds often have bridged or polymeric structures. The methyl derivatives of Li, Be and Al are found to be 3-D polymers, linear chains and dimeric respectively and despite the increase in RMM of the monomeric unit there is actually an increase in volatility.
| Compound | RMM of monomeric unit | Structure | Volatility |
|---|---|---|---|
| LiMe | 21.96 | 3D-polymer | infusible |
| BeMe2 | 39.08 | Linear chain | sublimes at 473 K |
| AlMe3 | 72.08 | Dimer | Melts at 288 K |
There has been some criticism of the term electron deficient since if a MO approach to the bonding is used then the bonding MO's derived from combination of the available atomic orbitals of suitable energy are generally full. Rundle (who determined the structure of BeMe2) is reported to have made the comment that:
There is no such thing as electron deficient compounds, only theory deficient chemists.
Stability of Organometallic compounds
The M-C bond energies for some methyl derivatives are shown in the Table below. Plotting these values against Atomic Number of the metal shows that there is a decrease down a Group. This behaviour is expected since there should be better orbital overlap between similar valence orbitals and this would decrease for the larger more diffuse elements lower down a Group.
| Me2M | D / kJ mol-1 | ΔHf / kJ mol-1 | BP /K | Me3M | D / kJ mol-1 | ΔHf / kJ mol-1 | BP /K | Me4M | D / kJ mol-1 | ΔHf / kJ mol-1 | BP /K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Me2Be | 490.2 | Me3B | 364.0 | -122.6 | 251.2 | Me4C | 347.3 | -167.4 | 283.2 | ||
| Me2Mg | Me3Al | 276.1 | -129.7 | 399.2 | Me4Si | 292.9 | -238.5 | 300.2 | |||
| Me2Zn | 175.7 | 54.8 | 317.2 | Me3Ga | 246.9 | -45 | 329.2 | Me4Ge | 246.9 | -71 | 316.2 |
| Me2Cd | 138.1 | 109.6 | 379.2 | Me3In | 171.5 | 409.2 | Me4Sn | 217.6 | -19.2 | 350.2 | |
| Me2Hg | 121.3 | 93.3 | 366.2 | Me3Tl | 420.2 | Me4Pb | 154.8 | 136.4 | 383.2 | ||
| Me3As | 230.1 | 15.5 | 325.2 | ||||||||
| Me3Sb | 217.6 | 31.0 | 352.2 | ||||||||
| Me3Bi | 142.3 | 192.9 | 383.2 |
Thermal Stability
In general terms thermodynamic stability means that the ΔG° is negative i.e. the energy of the products is more stable than that of the starting materials. Since little free energy data is available, it is often assumed that ΔH can be considered as a guide remembering that the entropies of gases are much larger than for liquids, which is again much larger than for solids and this can be taken into account as well.
if we take as an example the thermal decomposition of EtLi:
EtLi → LiH + CH2=CH2
ΔHf EtLi = -58.55 kJ mol-1
ΔHf CH2=CH2 = +52.40 kJ mol-1
ΔHf LiH = -90.45 kJ mol-1
so that the overall enthalpy change is: = (RHS - LHS) = 52.40 - 90.45 + 58.55
= +20.50 kJ mol-1
This therefore suggests that the data favours the stability of EtLi over the products. However, given that at room temperature the Entropy of gaseous ethylene (ethene) is high TΔS = +64.4 kJ mol-1 and the entropies for solids will be much smaller, then using ΔG = ΔH - TΔS it is likely that ΔG will be a sizable NEGATIVE value which would suggest that EtLi should be unstable.
Kinetic Stablity
Calculations of free energy would suggest that many organometallic compounds should be unstable. However, kinetic stability needs to be considered as well since if there is no low activation energy pathway for a reaction to proceed then it may be very slow.
Stability to Oxidation
All organometallic compounds are expected to be thermodynamically unstable with respect to oxidation to give metal oxide, carbon dioxide and water. Some are spectacularly so, being highly pyrophoric. In general organometallic compounds need to be handled under dry nitrogen or some other inert gas to avoid oxidation.
Stability to Hydrolysis
Hydrolysis of organometallic compounds often involves nucleophilic attack by water which is accentuated when there are low-lying empty orbitals on the metal atom. This is seen for Groups I, II and for Zn, Cd, Al, Ga etc and the speed of hydrolysis is dependent on the M-C bond polarity. For "Me3Al" rapid attack occurs whereas Me3B is unaffected at room temperature.
Classification of Synthetic Reactions
1. Elemental Reactions
a) with organic halides - the most important method
eg RX + M → RM + MX
b) with hydrocarbons
i)substitution
where the hydrocarbons are acidic
eg RC≡C-H + K → RC≡C-K + ½H2
eg R + K → RK + ½H2
ii) addition
eg Na + naphthalene → Na+ naphth- (C8H10)-
c) with other organometallics - transmetallation
eg M + M'R → MR + M'
2. Reactions with Element halides or salts
a) with hydrocarbons
eg RH + HgX2 → RHgX + HX
b) with other organometallics
eg MX + M'R → M'X + MR
3. Addition and Elimination Reactions
a) addition
eg -M-X + RHC=CH2 → M-C-C-X
Ph3SnH + PhCH=CH2 → Ph3SnCH2CH2.Ph
b) elimination
eg -M-A-B-C → -M-C + A=B
Hg(-OOC-R)2 + heat → HgR2 + 2CO2
References
1. "Inorganic Chemistry" - C. Housecroft and A.G. Sharpe, Prentice Hall, 3rd Ed., Dec 2007, ISBN13: 978-0131755536, ISBN10: 0131755536, Chapter 17.
2. "Chemistry of the Elements", Greenwood and Earnshaw, Elsevier.
Contributors and Attributions
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)

