Pressure
- Page ID
- 53592
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Describe the significance (and applications) of measuring pressure
What is Pressure?
Pressure is defined as force/area. For instance, the pressure from snow on a roof would be the weight of the snow divided by the area of the roof. In chemistry, usually pressure comes from gases. When you blow up a balloon, you put gas inside. The gas molecules bump into each other and into the walls of the balloon. Although the force from each molecule bumping the balloon is very small, when you put enough air in, all the collisions add up and make the balloon stretch and get bigger.

The absence of pressure is called vacuum. For hundreds of years, people thought that vacuums were impossible and unnatural: "nature abhors a vacuum." This isn't actually true. Miners had noticed that they could only pump water about 10 m up a pipe; the vacuum at the top from the pump wasn't strong enough to lift the water any higher. In 1641, Berti tried an experiment. He built a giant pipe about 13 m tall next to his house and filled it with water. The top of the tube was sealed. The bottom was in a big bucket. The he made a hole at the bottom so water could flow out of the tube into the bucket. Water flowed down until the water column was about 10.3 m high. Then it stopped.
Torricelli, later a companion of Galileo, experimented further. He noticed that a column of mercury (similar to the column of water in Berti's experiment) will be 760 mm high. Water has a density of 1 g/mL, while mercury has a density of 13.5 g/mL. 10.3 m/13.5 = 763 mm. From this he concluded that the column of liquid is held up not by pull from the vacuum above but by push from the weight of air (the atmosphere) on the open surface of the bucket. The weight of the mercury column, water column and air column were the same. Traditionally, pressure was measured using a barometer, which originally was just a tube with a column of liquid. The greater the height of the liquid, the higher the external pressure. Barometers can be used to measure pressure, which can be used to predict weather. Often low pressure means rain or storm is coming.
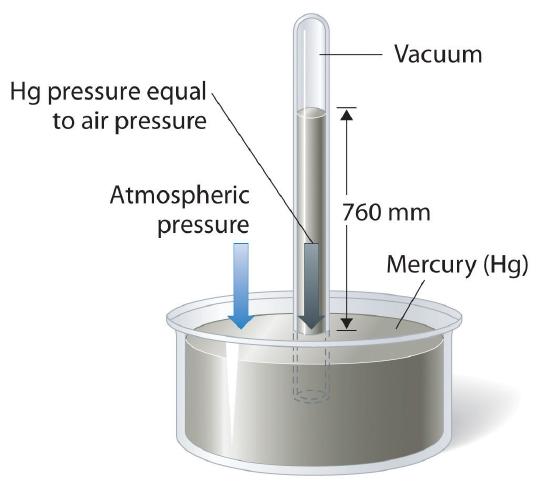
How do we Measure Pressure?
We can use a column of liquid, or other methods. One involves a small flexible container with a vacuum inside it, prevented from collapsing by springs. It expands or shrinks depending on the pressure, and this can be measured. Now there are also even smaller, simpler electrical barometers.
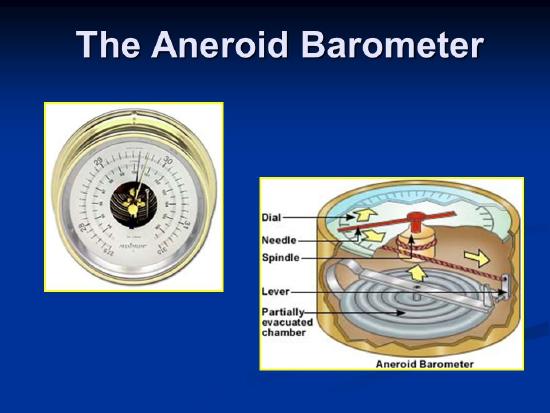
What Units should we use?
There are a crazy number of units for pressure! A traditional unit is the torr or mmHg. This just means the height of a column of mercury. Atmospheric pressure is about 760 torr or mmHg, as discussed above. You might also see mmH2O, which uses the same concept, but in this case because water is less dense than mercury, atmospheric pressure is about 10.3 mH2O.
There are some more modern units also. In SI, we use Pascals: 1 Pa = N/m2. Often it's more convenient to use bar: 1 bar = 105 Pa. Another unit you may have used before is the atmosphere: 1 atm = 1.01325 bar = 760 torr. The atm is close to the average atmospheric pressure.
When you use pressure units, because there are so many of them, you should be extra careful to check your units and make sure they cancel properly (see Dimensional Analysis for more). If you are using SI units in the rest of your calculation (like forces in N, mass in kg, etc) your pressure will probably come out in Pa.
How do we Control Pressure?
Often we want to control pressures, making them either higher or lower than atmospheric pressure. For instance, in chemical industry, many reactions are run at high pressures. In the lab, we might use low pressure to pull a liquid through a filter or evaporate a solvent. We also can use vacuum techniques to do "air-free" chemistry, if we want to study molecules that react with water or air, by removing all the air from our containers before adding the chemicals. Many important instruments used in physics and chemistry, like electron microscopes, only work under vacuum.
To make a vacuum, we usually use a pump to remove air. We can't make a perfect vacuum that doesn't have any gas molecules, but we can reduce the number of molecules to quite low levels. To get ultrahigh vacuum, or even just high vacuum, we might use 2 or more different types of pumps. Some pumps work by repeatedly expanding a volume, so that the gas expands into the bigger space, then gets separated from the area that is being evacuated. (See some images on Wikipedia.) Or we could absorb the gas molecules onto a surface to remove them the space being evacuated. Normal vacuum cleaners used in homes might be at 0.2 atm, while ultrahigh vacuum in a lab might be 100 nPa.
To make very high pressures, scientists sometimes use diamond anvils. For instance, geochemists who study how rocks form might put a bit of water and mineral powder between the tips of two small, pointy diamonds (just like you might see in an engagement ring). Then they push the diamonds together, and the force gets concentrated onto just the tiny tips of the diamonds, so the pressure is huge, like 3 million atm. And because the diamonds are clear, the scientists can watch what happens right through the diamond!
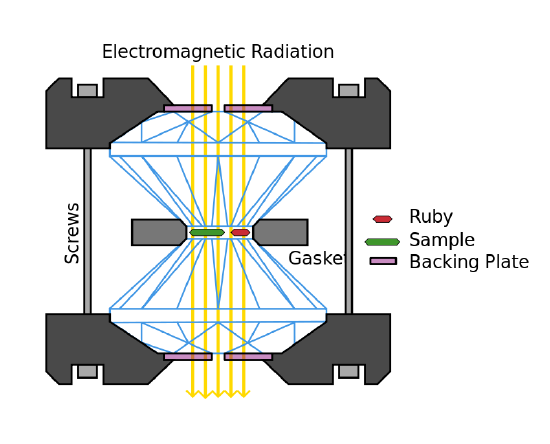
Figure 3: Cross section of a Diamond Anvil Cell. The following things are included: The two diamonds in between which the pressure is created. The sample. A Ruby which is usually used as a pressure indicator. The Gasket which seals the sample chamber The casing with the screws. Tightening of the screws moves the casings and the diamond closer together and builds pressure. The backing plate which holds the diamond in place. Electromagnetic rays which pass through the sample chamber to allow measurements. (CC-SA-BY-3.0; Tobias1984)
Contributors and Attributions
Emily V Eames (City College of San Francisco)

