3.4: A Molecular View of Elements and Compounds
- Page ID
- 397414
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Classify substances as atomic elements, molecular elements, molecular compounds, or ionic compounds.
Elements
There are lots of elements, and you don't need to memorize them all. Here are a few that you should learn right now, though, because they are common or important so that you won't be confused when they are mentioned later. They are organized by their type.
Atomic Elements
Most elements exist with individual atoms as their basic unit. It is assumed that there is only one atom in a formula if there is no numerical subscript on the right side of an element’s symbol.
- Non-metals - All Noble Gases. Example:
- Helium (He): named after the sun, because it was discovered in the sun before being discovered on Earth (we'll explain how later); it doesn't react with anything.
- Metals: soft or hard, light or heavy, usually solid electric conductors. Examples:
- Lithium (Li): the lightest alkali, used in batteries and anti-depressants
- Lead (Pb, from Latin plumbum): sweet-tasting toxic metal, now used to shield radiation and in bullets, among other uses
- Iron (Fe, from Latin ferrum): most abundant element on earth, essential in steel, with complex reaction properties essential to life
- Copper (Cu, from Latin cuprum): less reactive metal, with characteristic colors, commonly used in coins and electronics
- Gold (Au, from Latin aurum): used since ancient times in coins and jewelry, to color stained glass, also in dentistry and other applications
- Mercury (Hg, from Latin hydrargyrum): also called quicksilver, because it is a silver liquid, it is toxic but very important in the history of science; used in thermometers.
Molecular Elements
Many substances exist as two or more atoms connected together so strongly that they behave as a single particle. These multi-atom combinations are called molecules. A molecule is the smallest part of a substance that has the physical and chemical properties of that substance. In some respects, a molecule is similar to an atom. A molecule, however, is composed of more than one atom.
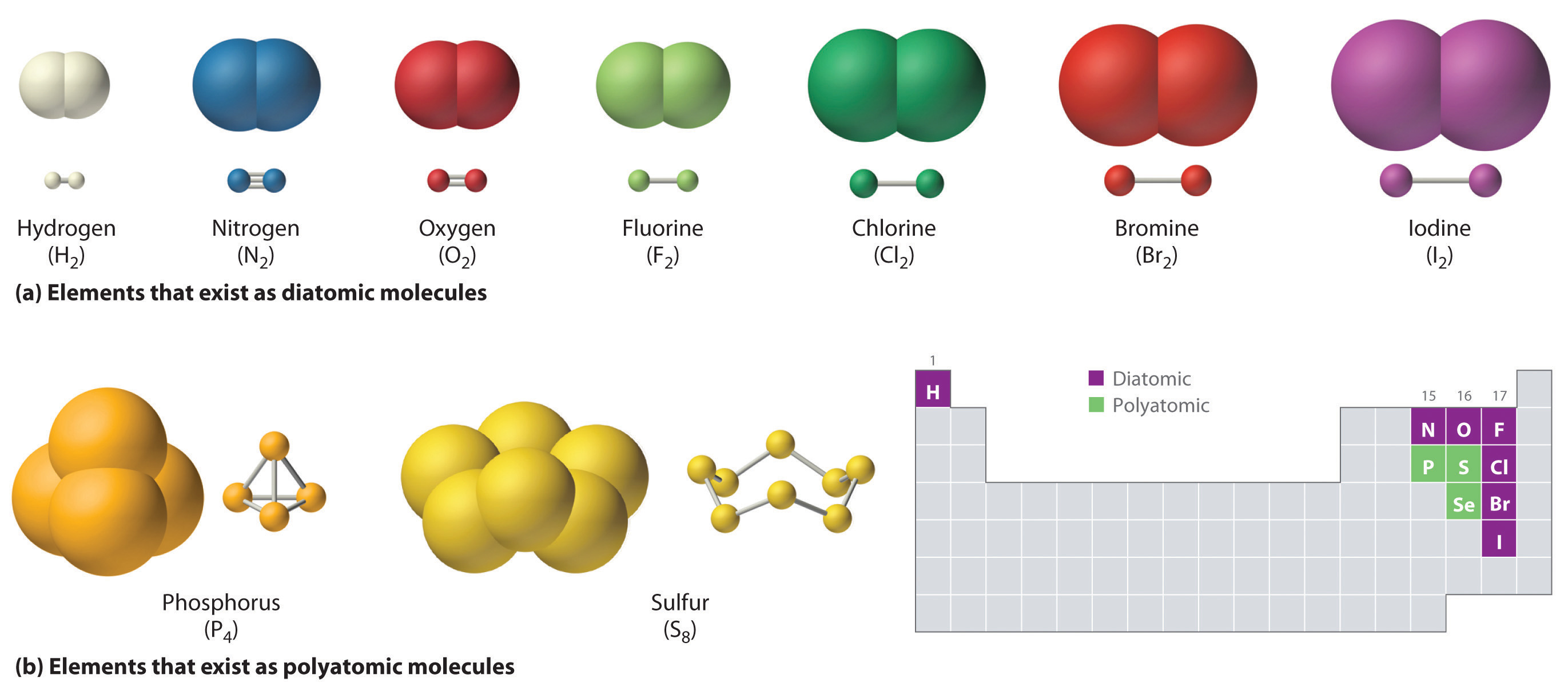
Some elements exist naturally as molecules. For example, hydrogen and oxygen exist as two-atom molecules. Other elements also exist naturally as diatomic molecules—a molecule with only two atoms (Figure \(\PageIndex{1}\)). As with any molecule, these elements are labeled with a molecular formula, a formal listing of what and how many atoms are in a molecule. (Sometimes, only the word formula is used, and its meaning is inferred from the context.)
For example, the molecular formula for elemental hydrogen is H2, with H being the symbol for hydrogen and the subscript 2 implying that there are two atoms of this element in the molecule. Other diatomic elements have similar formulas: O2, N2, and Halogens F2, Cl2, Br2, and I2.
Other elements exist as molecules—for example, sulfur normally exists as an eight-atom molecule, S8, while phosphorus exists as a four-atom molecule, P4 (Figure \(\PageIndex{1}\)).
Figure \(\PageIndex{1}\) shows two examples of how molecules will be represented in this text. An atom is represented by a small ball or sphere, which generally indicates where the nucleus is in the molecule. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a molecule. This connection is called a chemical bond.
Ionic Compounds
The elements in the periodic table are divided into specific groupings; metals, non-metals, metalloids, and so on. These groupings are largely based on physical properties and the tendency of the various elements to bond with other elements by forming either an ionic or a covalent bond. As a general rule of thumb, compounds involving metal binding with either a non-metal or a metalloids will display ionic bonding. Thus, the compound formed from sodium and chlorine will be ionic (a metal and a non-metal). The basic unit of ionic compounds is the formula unit.
Molecular Compounds
Compounds composed of only non-metals or semi-metals with non-metals will display covalent bonding and be classified as molecular compounds. Nitrogen monoxide (NO) will be a covalently bound molecule (two non-metals), and silicon dioxide (SiO2) will also be a covalently bound molecule (a semi-metal and a non-metal). The basic unit of molecular compounds is the molecule.
Provide the classification (i.e. atomic element, molecular element, molecular compound, or ionic compound) of each substance.
- Fe
- PCl3
- LiBr
- P4
- oxygen gas
Solution
- Fe (iron) is an element that is represented with no subscript, so it is an atomic element.
- PCl3 is made up of two nonmetals, so it is a molecular compound.
- LiBr is made up of lithium, a metal, and bromine, a nonmetal, so it is an ionic compound.
- P4 is a substance that is made up of four atoms of the same element, so it is a molecular element.
- The formula for oxygen gas is O2 so it is a molecular element.
Provide the classification (i.e. atomic element, molecular element, molecular compound, or ionic compound) of each substance.
- I2
- He
- H2O
- Al
- CuCl
- Answer a:
- molecular element
- Answer b:
- atomic element
- Answer c:
- molecular compound
- Answer d:
- atomic element
- Answer e:
- ionic compound

