Learning Objective
recognize and classify the common functional groups of organic chemistry (alkanes, alkenes, alkynes, alkyl halides, alcohols, amines, ethers, aldehydes, ketones, carboxylic acids, esters, and amides
Functional groups in organic compounds
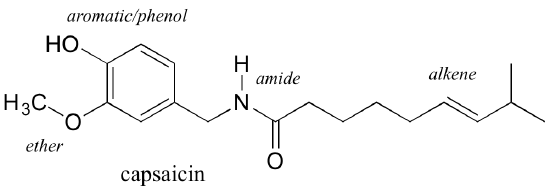
Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. The structure of capsaicin, the compound responsible for the heat in peppers, incorporates several functional groups, labeled in the figure below and explained throughout this section.
As we progress in our study of organic chemistry, it will become extremely important to be able to quickly recognize the most common functional groups, because they are the key structural elements that define how organic molecules react . For now, we will only worry about drawing and recognizing each functional group, as depicted by Lewis and line structures. Much of the remainder of your study of organic chemistry will be taken up with learning about how the different functional groups behave in organic reactions.
The 'default' in organic chemistry (essentially, the lack of any functional groups) is given the term alkane , characterized by single bonds between carbon and carbon, or between carbon and hydrogen. Methane, CH4 , is the natural gas you may burn in your furnace. Octane, C8 H18 , is a component of gasoline.
Alkanes
Alkenes (sometimes called olefins ) have carbon-carbon double bonds, and alkynes have carbon-carbon triple bonds. Ethene, the simplest alkene example, is a gas that serves as a cellular signal in fruits to stimulate ripening. (If you want bananas to ripen quickly, put them in a paper bag along with an apple - the apple emits ethene gas, setting off the ripening process in the bananas). Ethyne, commonly called acetylene, is used as a fuel in welding blow torches.
Alkenes and alkynes
Alkenes have trigonal planar electron geometry while alkynes have linear geometry. Furthermore, many alkenes can take two geometric forms: cis trans cis and trans forms of a given alkene are different molecules with different physical properties there is a very high energy barrier to rotation about a double bond. In the example below, the difference between cis and trans alkenes is readily apparent.
Alkanes, alkenes, and alkynes are all classified as hydrocarbons , because they are composed solely of carbon and hydrogen atoms. Alkanes are said to be saturated hydrocarbons , because the carbons are bonded to the maximum possible number of hydrogens - in other words, they are saturated with hydrogen atoms. The double and triple-bonded carbons in alkenes and alkynes have fewer hydrogen atoms bonded to them - they are thus referred to as unsaturated hydrocarbons . As we will see in chapter 15, hydrogen can be added to double and triple bonds, in a type of reaction called 'hydrogenation'.
The aromatic group is exemplified by benzene (which used to be a commonly used solvent on the organic lab, but which was shown to be carcinogenic), and naphthalene, a compound with a distinctive 'mothball' smell. Aromatic groups are planar (flat) ring structures, and are widespread in nature. We will learn more about the structure and reactions of aromatic groups in chapters 2 and 14.
Aromatics
When the carbon of an alkane is bonded to one or more halogens, the group is referred to as a alkyl halide or haloalkane . Chloroform is a useful solvent in the laboratory, and was one of the earlier anesthetic drugs used in surgery. Chlorodifluoromethane was used as a refrigerant and in aerosol sprays until the late twentieth century, but its use was discontinued after it was found to have harmful effects on the ozone layer. Bromoethane is a simple alkyl halide often used in organic synthesis. Alkyl halides groups are quite rare in biomolecules.
Haloalkanes In the alcohol functional group, a carbon is single-bonded to an OH group (the OH group, by itself, is referred to as a hydroxyl ). Except for methanol, all alcohols can be classified as primary, secondary, or tertiary. In a primary alcohol , the carbon bonded to the OH group is also bonded to only one other carbon. In a secondary alcohol and tertiary alcohol, the carbon is bonded to two or three other carbons, respectively. When the hydroxyl group is directly attached to an aromatic ring, the resulting group is called a phenol . The sulfur analog of an alcohol is called a thiol (from the Greek thio , for sulfur).
Alcohols, phenols, and thiols
Note that the definition of a phenol states that the hydroxyl oxygen must be directly attached to one of the carbons of the aromatic ring. The compound below, therefore, is not a phenol - it is a primary alcohol.
The distinction is important, because as we will see later, there is a significant difference in the reactivity of alcohols and phenols.
The deprotonated forms of alcohols, phenols, and thiols are called alkoxides , phenolates , and thiolates , respectively. A protonated alcohol is an oxonium ion.
In an ether functional group, a central oxygen is bonded to two carbons. Below is the structure of diethyl ether, a common laboratory solvent and also one of the first compounds to be used as an anesthetic during operations. The sulfur analog of an ether is called a thioether or sulfide .
Ethers and sulfides
Phosphate and its derivative functional groups are ubiquitous in biomolecules. Phosphate linked to a single organic group is called a phosphate ester ; when it has two links to organic groups it is called a phosphate diester . A linkage between two phosphates creates a phosphate anhydride .
Organic phosphates
There are a number of functional groups that contain a carbon-oxygen double bond, which is commonly referred to as a carbonyl . Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens.
A group with a carbon-nitrogen double bond is called an imine , or sometimes a Schiff base (in this book we will use the term 'imine'). The chemistry of aldehydes, ketones, and imines will be covered in chapter 10.
Aldehydes, ketones, and imines
When a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to an oxygen, nitrogen, or sulfur, the functional group is considered to be one of the ‘carboxylic acid derivatives ’, a designation that describes a set of related functional groups. The eponymous member of this family is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl group. The conjugate base of a carboxylic acid is a carboxylate . Other derivatives are carboxylic esters (usually just called 'esters' ), thioesters , amides , acyl phosphates , acid chlorides , and acid anhydrides . With the exception of acid chlorides and acid anhydrides, the carboxylic acid derivatives are very common in biological molecules and/or metabolic pathways, and their structure and reactivity will be discussed in detail in chapter 11.
Carboxylic acid derivatives
A single compound often contains several functional groups, particularly in biological organic chemistry. The six-carbon sugar molecules glucose and fructose, for example, contain aldehyde and ketone groups, respectively, and both contain five alcohol groups (a compound with several alcohol groups is often referred to as a ‘polyol’ ).
The hormone testosterone, the amino acid phenylalanine, and the glycolysis metabolite dihydroxyacetone phosphate all contain multiple functional groups, as labeled below.
While not in any way a complete list, this section has covered most of the important functional groups that we will encounter in biological organic chemistry.
Exercise:
1. Identify the functional groups (other than alkanes) in the following organic compounds. State whether alcohols and amines are primary, secondary, or tertiary.
Solution
1.
Amines are characterized by nitrogen atoms with single bonds to hydrogen and carbon. Just as there are primary, secondary, and tertiary alcohols, there are primary, secondary, and tertiary amines. Ammonia is a special case with no carbon atoms.
One of the most important properties of amines is that they are basic, and are readily protonated to form ammonium cations. In the case where a nitrogen has four bonds to carbon (which is somewhat unusual in biomolecules), it is called a quaternary ammonium ion.
Amines
Note: Do not be confused by how the terms 'primary', 'secondary', and 'tertiary' are applied to alcohols and amines - the definitions are different. In alcohols, what matters is how many other carbons the alcohol carbon is bonded to, while in amines, what matters is how many carbons the nitrogen is bonded to.
Finally, a nitrile group is characterized by a carbon triple-bonded to a nitrogen.
Nitriles
Exercise
2. Draw one example of each compound that includes the specified structural features. Be sure to designate the location of all non-zero formal charges. All atoms should have complete octets (phosphorus may exceed the octet rule). There are many possible correct answers for these, so be sure to check your structures with your instructor or tutor.
a) a compound with molecular formula C6 H11 NO that includes alkene, secondary amine, and primary alcohol functional groups
b) an ion with molecular formula C3 H5 O6 P 2- that includes aldehyde, secondary alcohol, and phosphate functional groups.
c) A compound with molecular formula C6 H9 NO that has an amide functional group, and does not have an alkene group.
Answer
2.