14.7: Polypeptides and Proteins
- Page ID
- 341985
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)


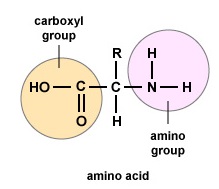 Figure \(\PageIndex{1}\): Amino Acids. Structure of an amino acid.
Figure \(\PageIndex{1}\): Amino Acids. Structure of an amino acid.
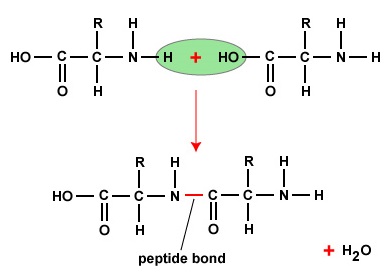 Figure \(\PageIndex{2}\): Peptide Bonds. A peptide bond forms when the amino group of one amino acid bonds to the carboxyl group of another amino acid.
Figure \(\PageIndex{2}\): Peptide Bonds. A peptide bond forms when the amino group of one amino acid bonds to the carboxyl group of another amino acid.
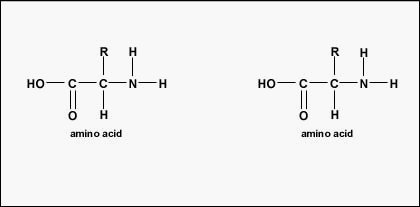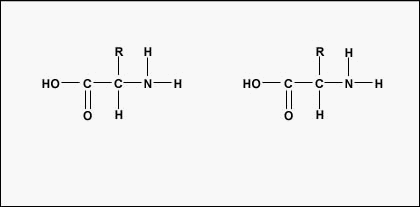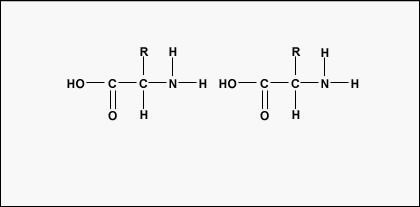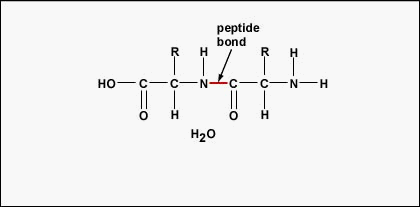 Figure \(\PageIndex{3}\): Formation of a Peptide Bond. A peptide bond forms when the amino group of one amino acid bonds to the carboxyl group of another amino acid.
Figure \(\PageIndex{3}\): Formation of a Peptide Bond. A peptide bond forms when the amino group of one amino acid bonds to the carboxyl group of another amino acid.
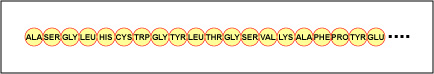 Figure \(\PageIndex{4}\): Primary Structure of a Protein or Polypeptide. The primary structure of a protein or polypeptide is the actual sequence of its amino acids. Primary structure is determined by the order of the deoxyribonucleotide bases in genes.
Figure \(\PageIndex{4}\): Primary Structure of a Protein or Polypeptide. The primary structure of a protein or polypeptide is the actual sequence of its amino acids. Primary structure is determined by the order of the deoxyribonucleotide bases in genes.
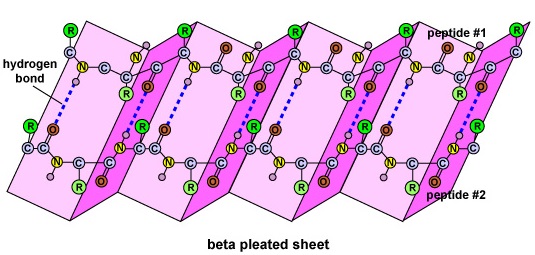 Figure \(\PageIndex{5}\): Secondary Structure of a Protein or Polypeptide. (left) The secondary structure of a protein or polypeptide is due to hydrogen bonds forming between an oxygen atom of one amino acid and a nitrogen atom of another. There are two possible types of secondary structure: an alpha helix and a beta sheet. In the case of an alpha helix, the hydrogen bonding causes the polypeptide to twist into a helix. With a beta sheet the hydrogen bonding enables the polypeptide to fold back and forth upon itself like a pleated sheet. (right) The secondary structure of a protein or polypeptide is due to hydrogen bonds forming between an oxygen atom of one amino acid and a nitrogen atom of another. There are two possible types of secondary structure: an alpha helix and a beta sheet. In the case of an alpha helix, the hydrogen bonding causes the polypeptide to twist into a helix. With a beta sheet the hydrogen bonding enables the polypeptide to fold back and forth upon itself like a pleated sheet.
Figure \(\PageIndex{5}\): Secondary Structure of a Protein or Polypeptide. (left) The secondary structure of a protein or polypeptide is due to hydrogen bonds forming between an oxygen atom of one amino acid and a nitrogen atom of another. There are two possible types of secondary structure: an alpha helix and a beta sheet. In the case of an alpha helix, the hydrogen bonding causes the polypeptide to twist into a helix. With a beta sheet the hydrogen bonding enables the polypeptide to fold back and forth upon itself like a pleated sheet. (right) The secondary structure of a protein or polypeptide is due to hydrogen bonds forming between an oxygen atom of one amino acid and a nitrogen atom of another. There are two possible types of secondary structure: an alpha helix and a beta sheet. In the case of an alpha helix, the hydrogen bonding causes the polypeptide to twist into a helix. With a beta sheet the hydrogen bonding enables the polypeptide to fold back and forth upon itself like a pleated sheet.
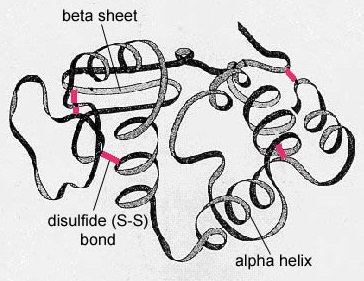 Figure \(\PageIndex{6}\): Tertiary Structure of a Protein or Polypeptide. In globular proteins such as enzymes, the long chain of amino acids becomes folded into a three-dimensional functional shape or tertiary structure. This is because certain amino acids with sulfhydryl or SH groups form disulfide (S-S) bonds with other amino acids in the same chain. Other interactions between R groups of amino acids such as hydrogen bonds, ionic bonds, covalent bonds, and hydrophobic interactions also contribute to the tertiary structure.
Figure \(\PageIndex{6}\): Tertiary Structure of a Protein or Polypeptide. In globular proteins such as enzymes, the long chain of amino acids becomes folded into a three-dimensional functional shape or tertiary structure. This is because certain amino acids with sulfhydryl or SH groups form disulfide (S-S) bonds with other amino acids in the same chain. Other interactions between R groups of amino acids such as hydrogen bonds, ionic bonds, covalent bonds, and hydrophobic interactions also contribute to the tertiary structure.
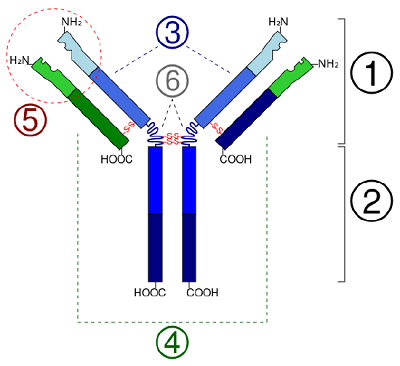 Figure \(\PageIndex{7}\): Quaternary Structure of a Protein. The quaternary structure of a protein is due to several polypeptides joining together, as in the case of antibody molecules. Schematic diagram of the basic unit of immunoglobulin (antibody) Fab Fc heavy chain (consist of VH, CH1, hinge, CH2 and CH3 regions: from N-term) light chain (consist of VL and CL regions: from N-term) antigen binding site hinge regions (*) -S-S- mean disulfide bonds. (CC-SA-BY 3.0;
Figure \(\PageIndex{7}\): Quaternary Structure of a Protein. The quaternary structure of a protein is due to several polypeptides joining together, as in the case of antibody molecules. Schematic diagram of the basic unit of immunoglobulin (antibody) Fab Fc heavy chain (consist of VH, CH1, hinge, CH2 and CH3 regions: from N-term) light chain (consist of VL and CL regions: from N-term) antigen binding site hinge regions (*) -S-S- mean disulfide bonds. (CC-SA-BY 3.0;