Dr. Tim Spector, Professor of Genetic Epidemiology at Kings College in London, knows a thing or two about twins. He should: as head of the Department of Twin Research at Kings College, Spector works with about 3500 pairs of identical twins, researching the influence of a person's genetic blueprint on everything from how likely they are to be obese, to whether or not they hold religious beliefs, to what kind of person they fall in love with. Anyone who is a twin, or has ever known a pair of identical twins, can attest to how remarkably similar they are to each other, even in the rare cases of adopted twins raised in separate homes. Dr. Spector, however, has over the course of his research become much more interested in how they are different.
![2018-08-10 23_44_43-OCBE 2016 Volume I (2).docx [Compatibility Mode] - Word (Product Activation Fai.png](https://chem.libretexts.org/@api/deki/files/140146/2018-08-10_23_44_43-OCBE_2016_Volume_I__(2).docx_%255BCompatibility_Mode%255D_-_Word_(Product_Activation_Fai.png?revision=1)
A recent article about Spector in the British newspaper The Guardian (June 1, 2013) begins with an introduction to two middle-aged twin sisters named Barbara and Christine, one of the pairs of twins in the Kings College study group. Although they were treated almost as a single person when growing up, with identical haircuts and clothes, the twins began to diverge in their teenage years as they gained the freedom to make their own choices. They began to dress quite differently, with Christine choosing much more conservative styles than Barbara. Christine describes herself as being self-conscious, while Barbara has always been more confident. Christine suffers from depression, but Barbara does not.
Given that they were born with the exact same DNA and were raised in the same home, where do these differences come from? In public debates about why people are the way they are, a catch phrase that often comes up is 'nature vs. nurture': people argue, in other words, about the relative influence of a person's genes vs. the influence of their environment. In Barbara and Christine's case, one would assume that the 'nature' is identical, and given that they grew up in the same house, the 'nurture' side of the equation should also be quite similar.
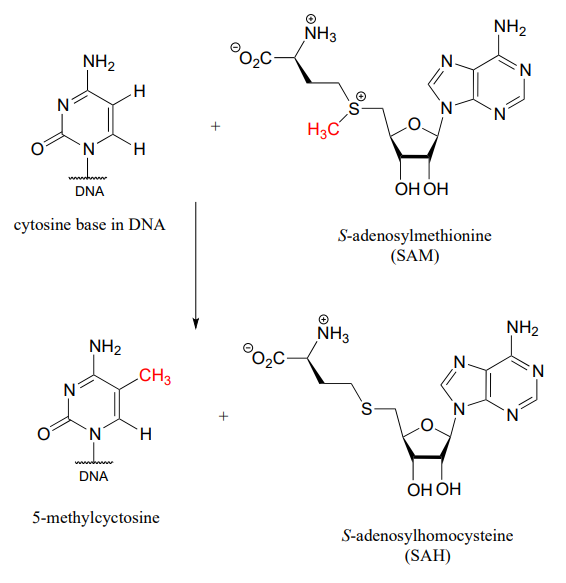
As it turns out, the 'nature' component may not be so identical after all. Based on his work with twins, Spector now thinks that subtle changes to Barbara's and Christine's DNA after conception - and indeed, throughout their lifetimes - may be a much more important determinant of their physical and psychological characteristics than was previously believed. As we age from infants to adulthood, some of our DNA bases are modified by methylation: in other words, a methyl (CH3) group replaces a hydrogen. In humans and other mammals, this mainly happens to cytosine (C) bases, while in bacteria it is mainly adenosine (A) bases which are methylated. The biomolecule that serves as the methyl group donor in both cases is called S-adenosyl methionine, or 'SAM' for short.
Methylation of cytosine
Methylation of adenine
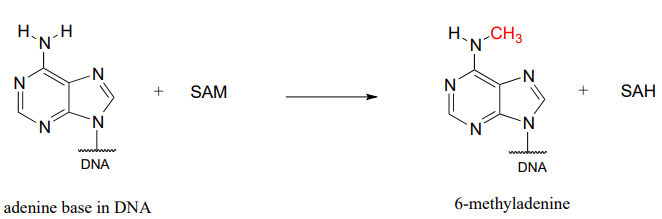
In mammals, gene methylation seems to occur in different patterns in different people - even in identical twins - in response to environmental factors. Methylation also seems to have the effect of amplifying or muting a gene's function, by altering how it interacts with regulatory proteins. The combined effect of many gene methylation events can be profound, as groups of interrelated genes are 'turned up' or 'turned down' in concert. Professor Spector thinks that the many differences between Barbara and Christine probably stem, at least in part, from differences in how their genes have been methylated over the course of their lives so far.
In this chapter, we delve for the first time into 'real' organic reactions, beyond the simple proton transfer events of Bronsted acid-base reactions that we looked at in chapter 7. The methylation of DNA is an excellent example of a type of organic reaction called nucleophilic substitution, to which we were introduced briefly in chapter 6 as a model for learning about some of the fundamental concepts of organic reactivity. Now we will delve more deeply into three crucial players in this bond-forming and bond-breaking process: the nucleophile, the electrophile, and the leaving group. In doing so, we will get a chance to practice and refine our skills in drawing organic reaction mechanisms using the curved arrow formality, and we will think about what a transition state and a reactive intermediate of a reaction might look like, and how the structure of these species determines the regiochemical and stereochemical outcome of a nucleophilic substitution reaction. Perhaps, in the time spent working on this chapter, some of the cytosines in your DNA will undergo nucleophilic substitution reactions to become methylated - and who knows how this will influence who you go on to become?



![2018-08-10 23_44_43-OCBE 2016 Volume I (2).docx [Compatibility Mode] - Word (Product Activation Fai.png](https://chem.libretexts.org/@api/deki/files/140146/2018-08-10_23_44_43-OCBE_2016_Volume_I__(2).docx_%255BCompatibility_Mode%255D_-_Word_(Product_Activation_Fai.png?revision=1)

