5.2.6: Protein isolation and enzyme assay
- Page ID
- 242467
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Lab #11: Isolating gluten from flour
adapted from J. Chem. Educ., DOI:10.1021/acs.jchemed.5b00311
Gluten — a mixture of glutenin and gliadin proteins of different molecular weights — is well known for being present in wheat and wheat products. However, people with gluten intolerance or Celiac-Sprue disease cannot properly digest such wheat products and so must avoid eating them altogether. Alternatives to gluten from common hybrid wheat strains are therefore of interest. In this activity, you will isolate gluten from flour and study some of its properties.
Background
As gluten intolerance and Celiac-Sprue disease have become more prominent in recent years, researchers have been exploring methods to cure or avert the problems caused by gluten in the modern diet. Celiac-Sprue is a recognized problem in which patients cannot properly digest gluten and their intestines gradually lose the ability to absorb nutrients. Currently, the best therapeutic strategy for either problem involves a total elimination of gluten from the diet. Due to the prevalence of gluten products and the likelihood for cross-contamination into other foods, gluten avoidance is difficult and inconvenient. A greater knowledge of the specific components of modern wheat that cause disease could allow the creation of new wheat strains or food products that do not cause these adverse reactions.
Many varieties of wheat have been directly evolved to have desirable characteristics to give bread its airy structure: its ability to trap carbon dioxide from fermenting yeasts. Different types of grain are have different natural levels of gluten — a mixture of glutenin and gliadin proteins of different molecular weights that give breads their structure.
Procedure
You will need some flour for this experiment. Pancake mix (the one where you have to add eggs, i.e. not a "complete" mixture) should work as well. If you have a contact-allergy to gluten, you should not do this activity. If all you have available is gluten-free flour, you won't be able to do this lab.
Transfer a table spoon of flour into a snack-size zip lock bag, add a teaspoon of water and close the bag (if you just have a generic spoon available, take three spoons of flour and one of water). Mix ("knead") the mixture until you get dough-like consistency - make sure all the flour gets wet. Leave the bag for at least 30 minutes to allow the gluten network to continue to develop.
Rinse the starch (and chaff, if you stared with whole wheat flour) away from the gluten with water. Using about 10 mL of water (two teaspoons) for each wash has worked in the past. You can do the wash step in the bag, carefully mixing the dough with the water, and pouring out the liquid. Save the initial rinse for a positive control in a clean container; the method for testing for starch is addition of a drop of iodine test reagent (stored in a 1.5 mL tube wrapped in aluminum foil in your kit). After a few more rinses (at least four total), collect the wash in one larger beaker, test a small amount of the latest rinse water for starch content and continue to rinse until the starch test is negative. Squeeze as much water as possible out of your sample.
Describe the properties of the gluten sample. Does it dissolve in water? How would you describe its consistency? You can handle the gluten with your hands - how "stretchy" is it? Take a tiny amount of the gluten between thumb and fingers, add some dish washing or hand washing detergent, and test whether you can dissolve the gluten while rolling and "smooshing" the gluten blob between thumb and fingers.
Lab #12: Enzyme assay (amylase)
Many diagnostic tests are based on enzyme activity. Either the test will determine the concentration of an enzyme in a sample, or it will indirectly determine the concentration of a small molecule in a sample, through a so-called enzyme-linked test (e.g. ELIZA-type test). Today, we will test the activity of the enzyme amylase.
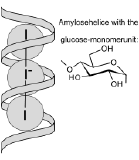
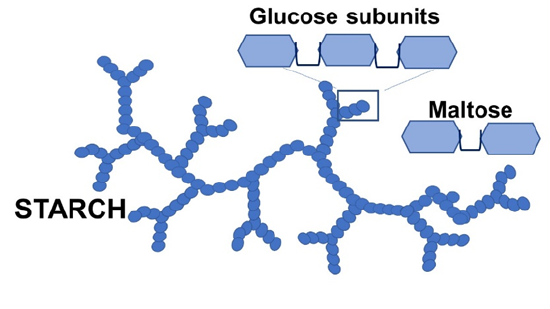 Amylase catalyzes the hydrolysis of starch (see figure on the left), made up of glucose building blocks. The product is maltose, a disaccharide. In the activity, we will hydrolize a starch sample with amylase. As the reaction proceeds, we will test for the presence of starch by adding a mixture of potassium iodide and iodine (KI and I2). These form linear I3- and I5- ions embedded in the spiral-staircase structure of starch, with a dark blue color (see sketch on the right. Once the starch is digested by the enzyme, the color is no longer observed.
Amylase catalyzes the hydrolysis of starch (see figure on the left), made up of glucose building blocks. The product is maltose, a disaccharide. In the activity, we will hydrolize a starch sample with amylase. As the reaction proceeds, we will test for the presence of starch by adding a mixture of potassium iodide and iodine (KI and I2). These form linear I3- and I5- ions embedded in the spiral-staircase structure of starch, with a dark blue color (see sketch on the right. Once the starch is digested by the enzyme, the color is no longer observed.
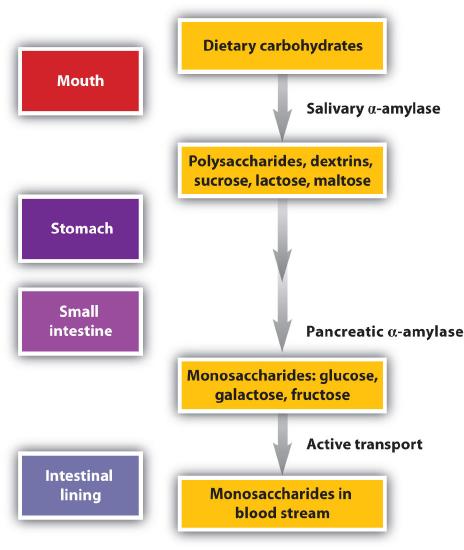 Our amylase sample will be obtained without purification from human saliva (the easiest source is your own saliva). Starch starts is broken down into disaccharides in the mouth and in the stomach, and further hydrolized to glucose in the small intestine.
Our amylase sample will be obtained without purification from human saliva (the easiest source is your own saliva). Starch starts is broken down into disaccharides in the mouth and in the stomach, and further hydrolized to glucose in the small intestine.
Task 1: Basic enzyme assay
You will need starch solution (clear or slightly turbid solution prepared by adding half a teaspoon of corn starch to one cup of water and heating in the microwave or on the stove top) and the iodine stop solution (yellowish brownish solution, wrapped in foil to keep out light) for this experiment, two clean empty 1.5 mL tubes, a bowl to collect a small amount of saliva (we need 20 µL), both transfer pipettes and a flat surface to test for starch (the snack-sized zip lock bag works well).
Use one transfer pipette to handle the iodine stop solution and the saliva, while using the other one to handle the starch solution (you don't want to contaminate the starch stock solution with amylase). Place eight 20 µL drops of iodine stop solution on (not in) the zip lock bag, keeping about an inch of distance between drops. Clean the pipette with water. Collect some saliva in a clean bowl, and transfer 20 µL of it into a clean 1.5 mL tube. To this, add 250 µL water and mix gently, then close the lid. This is your "enzyme stock solution".
With the other - amylase-free - pipette, transfer 500 µL starch solution into a second clean 1.5 mL tube. Start a timer after adding 100 µL of your enzyme stock solution (with the first pipette you used previously to make this stock). Incubate at room temperature, removing 20 µL samples at intervals (e.g. 10 seconds, 1, 2, 5, 10 min) and mixing each with with a fresh drop of stop solution on the zip lock bag. Samples taken at earlier times will be blue, while samples taken at later times will be yellowish if the enzyme has broken down most of the starch.
To analyze your results, compare the colors to the standards shown below. These are starch solutions mixed with iodine stop solution in the absence of enzyme. The first sample on the left shows undiluted starch solution, while the remainder are 1/4, 1/16, 1/64 dilution, and on the far right, no starch at all. The undiluted starch solution contains about 0.5 g starch per 100 mL solution, or 0.5%. The standards show that 0.03% starch is still detectable, while 0.008% is below the level of detection in this assay. In other words, if your sample looks like negative control, almost all of the starch has been digested. If, on the other hand, it looks as dark as the first (left-most) standard below, no or hardly any starch has been digested.

If you let the samples sit in the light for a while, the brownish color of the iodine stop solution fades while the blackish blue color of the starch : I3- complex is slightly more stable. This is because the free I3- species undergoes photo-degradation (which is also why the reagent was stored in aluminum foil).

Task 2: Change something
Repeat the basic assay, but change one parameter of the assay to answer a question of your choice. One possibility is to run the assay at a different temperature (cool down the reagents and water in a fridge to cool them down, or keep them in a glass of warm but not hot water). Other ideas are to do a positive or negative control (especially if there was some kind of unexpected result in task 1), or to change other parameters known to affect the rates of catalyzed reactions.

