2.4.1.1: A1. Monosaccharides Structures
- Page ID
- 238075
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Simple sugars can be defined as polyhydroxy-aldehydes or ketones. Hence the simplest sugars contain at least three carbons. The most common are the aldo- and keto-trioses, tetroses, pentoses, and hexoses. The simplest 3C sugars are glyceraldehye and dihydroxyacetone.
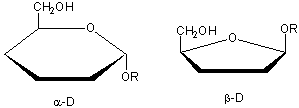
Glucose, an aldo-hexose, is a central sugar in metabolism. It and other 5 and 6C sugars can cyclize through intramolecular nucleophilic attack of one of the OH's on the carbonyl C of the aldehyde or ketone. Such intramolecular reactions occur if stable 5 or 6 member rings can form. The resulting rings are labeled furanose (5 member) or pyranose (6 member) based on their similarity to furan and pyran. On nucleophilic attack to form the ring, the carbonyl O becomes an OH which points either below the ring (a anomer) or above the ring (b anomer).
Figure: Sugar Ring Formation and Representations
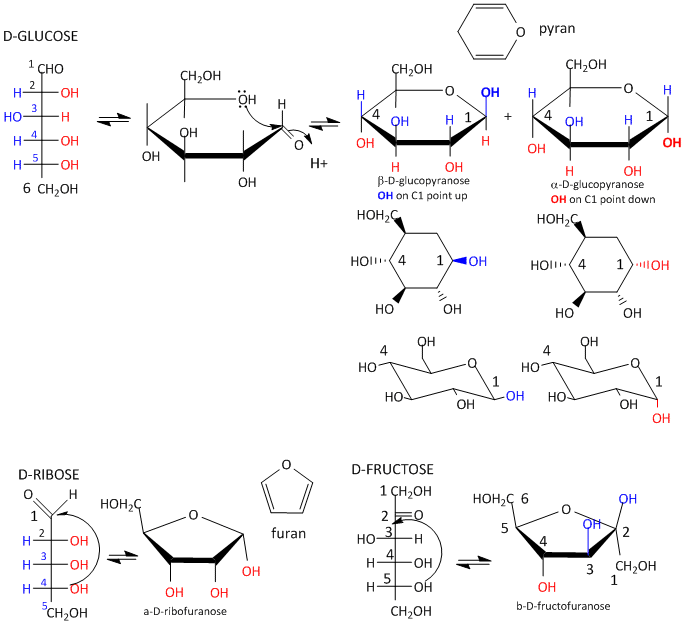
Monosaccharides in solution exist as equilibrium mixtures of the straight and cyclic forms. In solution, glucose (Glc) is mostly in the pyranose form, fructose is 67% pyranose and 33% furanose, and ribose is 75% furanose and 25% pyranose. However, in polysaccharides, Glc is exclusively pyranose and fructose and ribose are furanoses.
Sugars can be drawn in the straight chain form as either Fisher projections or perspective structural formulas.
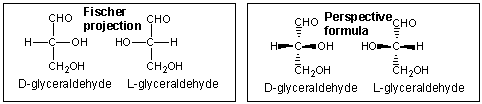
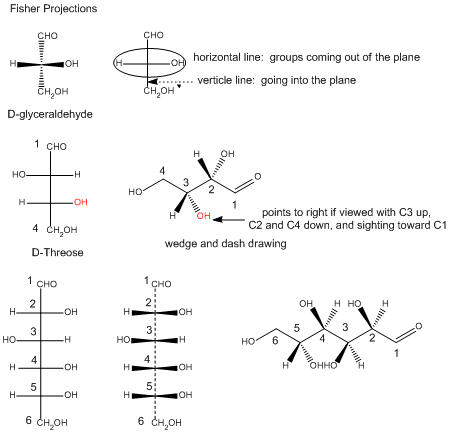
In the Fisher projection, the vertical bonds point down into the plane of the paper. That's easy to visualize for 3C molecules. but more complicated for bigger molecules. For those draw a wedge and dash line drawing of the molecule. When determining the orientation of the OHs on each C, orient the wedge and dash drawing in your mind so that the C atoms adjacent to the one of interest are pointing down. Sighting towards the carbonyl C, if the OH is pointing to the right in the Fisher project, it should be pointing to the right in the wedge and dash drawing, as shown below for D-erthyrose and D-glucose.
Figure: Orienting OH groups in wedge and dashing drawings of simple straight chain sugars
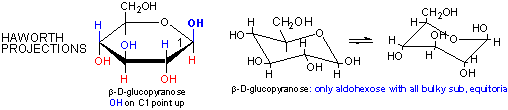
Cyclic forms can be drawn either as the Haworth projections, which shows the molecule as cyclic and planar with substituents above or below the ring) or the more plausible bent forms (showing Glc in the chair or boat conformations, for example). b-D-glucopyranose is the only aldohexose which can be drawn with all its bulky substituents (OH and CH2OH) in equatorial positions, which probably accounts for its widespread prevalence in nature.
 chair and boat conformations of cyclohexane (not functional on many browsers)
chair and boat conformations of cyclohexane (not functional on many browsers)
Haworth projections are more realistic than the Fisher projections, but you should be able to draw both structures. In general, if a substituent points to the right in the Fisher structure, it points down in the Haworth. if it points left, it points up. In general, the OH on the a-anomer points down (ants down) while on the b-anomer it points up (butterflies up).
![]()
![]() Jmol: Fisher to Ring Structures of Glucose
Jmol: Fisher to Ring Structures of Glucose
Figure: A more rigorous view of the relationship between the anomeric OH and the OH on the last chiral C of a sugar.
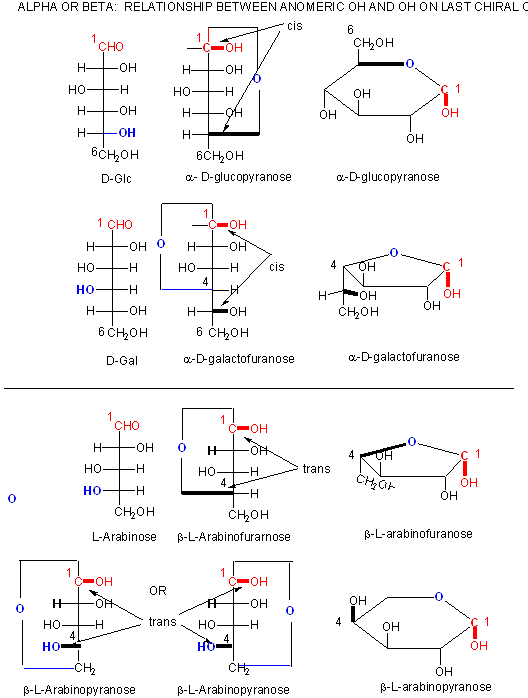
In the Haworth projections, the bulky R group of the next carbon after the carbon whose OH group engaged in a nucleop hilic attach on the carbonyl carbon to form the ring O is pointed up if the OH engaged in the attach was on the right hand side in the straight chain Fisher diagram (as in a-D-glucopyranose above when the CH2OH group is up) but is pointed down if the OH engaged in the attach was on the left hand side in the straight chain Fisher diagram (as in a-D-galactofuranose above when the (CHOH)CH2OH group is down). The rest of the OH groups still follow the simple rule that if they are pointing to the right in the Fisher straight chain form, they point down in the Haworth form.
![]() Jmol: Updated b-D-glucopyranose Jmol14 (Java) | JSMol (HTML5)
Jmol: Updated b-D-glucopyranose Jmol14 (Java) | JSMol (HTML5)
The most common monosaccharides (other than glyceraldehyde and dihydroxyacetone) which you need to know are shown below.
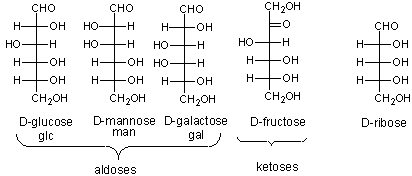
The mirror image of D-Glc is L-Glc. For common sugars, the prefix D and L refer to the center of asymmetry most remote from the aldehyde or ketone. By convention, all chiral centers are related to D- glyceraldehyde, so sugar isomers related to D-glyceraldehyde at their last asymmetric center are D sugars.


