11.7: Organic Compounds with Functional Groups
- Page ID
- 83153
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
-
to describe functional groups and explain why they are useful in the study of organic chemistry.
With over twenty million known organic compounds in existence, it would be very challenging to memorize chemical reactions for each one. Fortunately, molecules with similar functional groups tend to undergo similar reactions. A functional group is defined as an atom or group of atoms within a molecule that has similar chemical properties whenever it appears in various compounds. Even if other parts of the molecule are quite different, certain functional groups tend to react in certain ways.
We've already looked at alkanes, but they are generally unreactive. We primarily use alkanes as a source of energy when they are combusted. While the majority of functional groups involve atoms other than carbon and hydrogen, we will also look at some that include only carbon and hydrogen. Some of the most common functional groups are presented in the following sections.
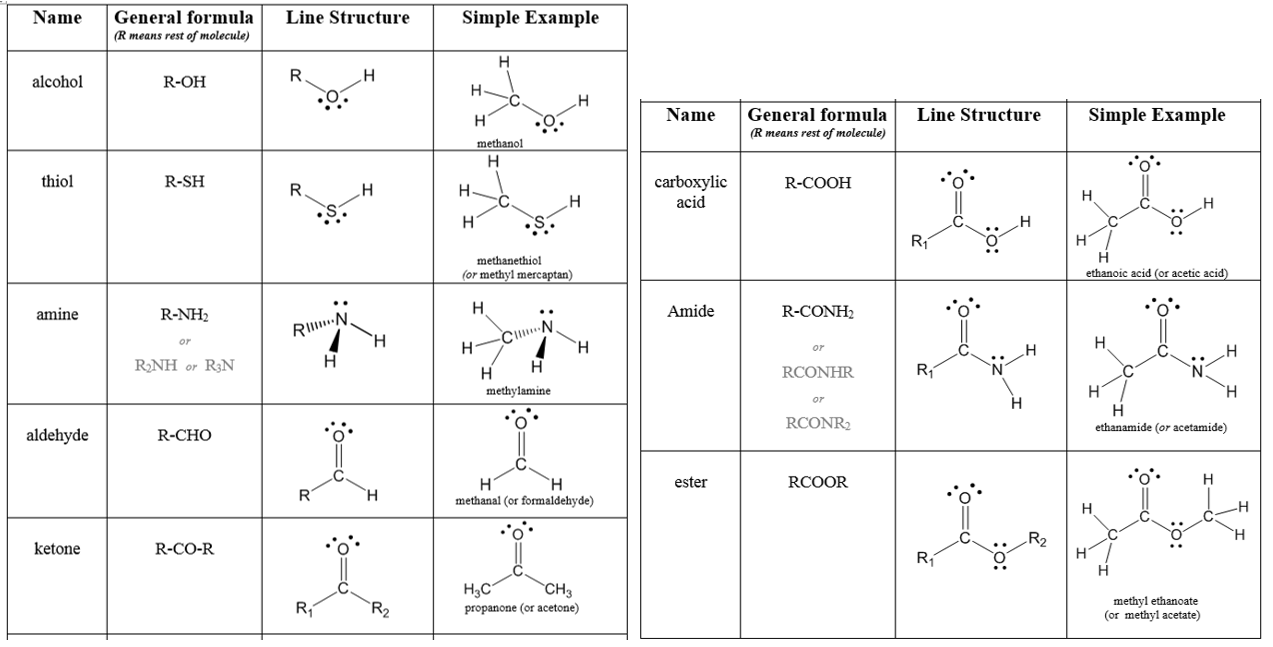
Organic molecules vary greatly in size and when focusing on functional groups, we want to direct our attention to the atoms involved in the functional group. As a result, the abbreviation R is used in some examples. The letter R is used in molecular structures to represent the “Rest of the molecule”. It consists of a group of carbon and hydrogen atoms of any size. It is used as an abbreviation since a group of carbon and hydrogen atoms does not affect the functionality of the compound. In some molecules, you will see R, R’, or R’’ which indicates that the R groups in the molecule can be different from one another. For example, R might be –CH2CH3 while R’ is –CH2CH2CH2CH3.
Previously, we considered several kinds of hydrocarbons. Now we examine some of the many organic compounds that contain functional groups with atoms beyond just carbon and hydrogen. A functional group is a specific structural arrangement of atoms or bonds that imparts a characteristic chemical reactivity to the molecule. If you understand the behavior of a particular functional group, you will know a great deal about the general properties of that class of compounds. In previous sections, we have looked at several classes of hydrocarbons. Saturated alkanes and cycloalkanes (with only single C-C and C-H bonds) are generally unreactive, primarily used as a source of energy when they are combusted. Unsaturated alkenes (hydrocarbons with C=C) and alkynes (hydrocarbonds with C≡C) are much more susceptible to addition reactions, in particular, and are used as precursors for the synthesis of many other compounds (like plastic polymers). Aromatic compounds (rings with alternating single and double bonds) are more stable than regular alkenes, thus less reactive, but commonly found in biological compounds (like steroids and medications). Here we add several more that include other atoms, like oxygen or nitrogen, in addition to carbon and hydrogen. Some common functional groups are listed in Table \(\PageIndex{1}\) below. The letter R represents the rest of the “Rest of the molecule”, typically a hydrocarbon chain or ring.
NOTE: There are others, but the organic functional groups introduced in this chapter are those most often discussed in later biochemistry chapters.
Alcohols
The alcohol functional group involves an oxygen atom that is bonded to one hydrogen atom and one carbon atom. The carbon atom will be part of a larger organic structure. One way to indicate a generic alcohol would be with the formula R−OH. R represents any organic fragment in which a carbon atom is directly bonded to the explicitly indicated functional group (in this case, OH). The R group is typically a chain of carbon atoms.
We are already familiar with several common alcohols. For example, ethanol (CH3CH2OH) is the alcohol present in alcoholic beverages. It is also widely used in the industrial manufacture of other chemicals. Methanol (CH3OH) is used as a gasoline additive or alternative. Additionally, methanol can be used to manufacture formaldehyde, which is employed in the production of plastics, paints, and other useful substances. Isopropanol is commonly known as rubbing alcohol. In addition to its industrial uses, isopropanol is used to clean various surfaces, including computer monitors, whiteboards, and even skin (e.g., before getting blood drawn).
Ethers
The ether functional group consists of an oxygen atom that forms single bonds with two carbon atoms. The general formula is R–O–R. Ethers are good solvents for other organic compounds because of their low reactivity. They readily dissolve nonpolar molecules. Diethyl ether (CH3CH2OCH2CH3) is perhaps the best known ether. It is widely used as a solvent and has been used as an inhalable anesthetic.
Although ethers themselves are relatively unreactive, they can be converted to peroxides after prolonged exposure to oxygen. Peroxides are very reactive and are often explosive at elevated temperatures. Many commercially available ethers come with a small amount of a peroxide scavenger dissolved in them to help prevent this type of safety hazard.
Thiol
The thiol functional group contains a sulfur atom bonded to a hydrogen atom. It is very similar to an alcohol functional group with the sulfur replacing the O (R–S–H).
Thiols are also called mercaptans which is derived from the Latin phrase for "capturing mercury" because of the strong bonds it forms with mercury-containing compounds. Some thiol compounds have a distinctive smell similar to rotten eggs. They are often added to natural gas, which itself has no odor, as a way to detect leaks since its odor can be detected by humans in very small amounts. A thiol group is also present in the amino acid cysteine which will be discussed later.
Amines
An amine consists of a nitrogen atom bonded to some combination of carbons and hydrogens.
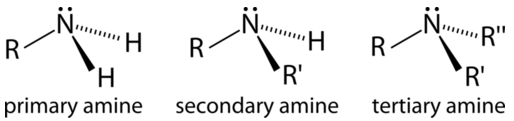
Like alcohols, amines can be classified as primary, secondary, or tertiary. However, the rules for assigning these categories are slightly different. In an alcohol, the oxygen atom is always bonded to exactly one carbon atom, so we look at the branching on the adjacent carbon, not the oxygen atom itself. In a neutral amine, the nitrogen can be bonded to one, two, or three carbon atoms, and this is how we decide whether it is called a primary, secondary, or tertiary amine.
Neutral amines are weak bases, because the lone pair on nitrogen can act as a proton acceptor. Many smaller amines have very strong and offensive odors. For example, the aptly-named compounds cadaverine and putrescine are foul-smelling amines, formed as a part of the decay process after death.
Amines serve a wide variety of uses. Diphenylamine acts as a stabilizer for certain types of explosives. Amines are found as components in some lubricating materials, in developers, and are a part of waterproofing textiles. Some amines, such as novocaine, are used as anesthetics. Many pharmaceutical compounds contain amines, including 8 of the 10 most prescribed medications in 2012.
Aldehydes
A very common structural component of organic structures is the carbonyl, which is simply a carbon atom and an oxygen atom connected by a double bond. The reactivity of carbonyls is primarily dictated by the polarization of the C=O bond, but the surrounding atoms also play a role in its specific reaction pathways. While carbonyl is a component of many functional groups, it is not itself a functional group.
General Formula of an Aldehyde, RCHO 
An aldehyde is a carbonyl in which the carbon atom is bonded to at least one hydrogen atom. The other group attached to the carbonyl may be an R-group or a hydrogen atom. Because the hydrogen atom is so small, the partial positive charge on the carbonyl carbon is very easy for other molecules to approach, making aldehydes a particularly reactive type of carbonyl. Aldehydes are versatile reactants for a wide variety of organic syntheses. Many aldehydes also have distinctive flavors and aromas. For example, the flavor of cinnamon is primarily due to the molecule cinnamaldehyde, and vanillin is the aldehyde most responsible for the smell and taste of vanilla extract.
A special aldehyde is the molecule in which the carbonyl is bonded to two hydrogen atoms. This molecule, called formaldehyde, has a wide variety of uses. By itself, it can be used as a tissue preservative or as a very harsh disinfectant. It is also used as a precursor to various materials, including plastics, resins, and other polymers.
Examples of molecules with aldehyde functional groups.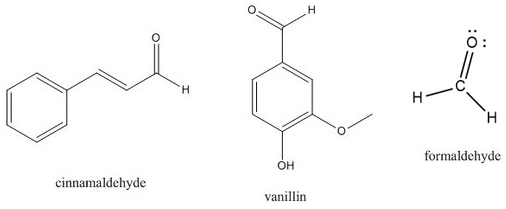
Ketones
General Formula of a Ketone 
A ketone involves a carbonyl in which the carbon atom makes single bonds with two R-groups. Ketones undergo most of the same reactions as aldehydes, but they tend to be slightly less reactive. The simplest ketone is acetone, in which the carbonyl carbon is bonded to two CH3 groups. This ketone is commonly used to remove fingernail polish and serves as an industrial solvent. Methyl ethyl ketone is used as a paint stripper and a solvent. Ketones are also used in the production of various polymers, either as a building block or as a solvent. The R-group in a ketone can be the same or different as seen in the example.
Two examples of ketones.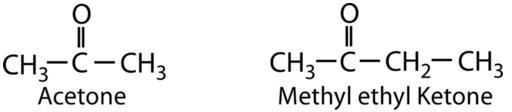
Carboxylic Acids
Carboxylic acids are another carbonyl-containing functional group, in which the carbon atom is bonded to an OHOH group on one side and either a carbon or hydrogen atom on the other.
General formula of a Carboxylic acid 
As the name implies, carboxylic acids are weak acids. An OH group that is directly connected to a carbonyl will ionize to a small extent when dissolved in water. The reason for this is the relative stability of the resulting anion. A carboxylate ion (see figure below), in which the negative charge is spread over two different oxygen atoms through resonance structures, is more stable than an isolated oxygen-centered anion. The carboxylic acid and carboxylate ion are interchangeable. Carboxylate ions are often present in amino acids.
Carboxylate ion 
Carboxylic acids are used in a variety of environments. Formic acid acts as a protective chemical for many stinging insects and plants. Acetic acid gives vinegar its characteristic smell and flavor and is a fundamental biological and industrial building block. Carboxylic acids with longer carbon chains (fatty acids) are used by animals as a way of storing energy and are widely used in the manufacture of soaps. Some compounds contain multiple carboxylic acids within a single molecule. For example, citric acid (three carboxyl groups) is especially abundant in citrus fruits and it used as a flavoring and preservative in many foods and beverages.
Carboxylic Acid Examples 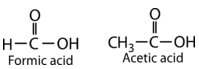
Esters
An ester is similar to a carboxylic acid, in that it contains a carbonyl where the carbon is bonded to one additional oxygen atom and one carbon or hydrogen atom. However, the second oxygen atom is bonded to another carbon instead of to an acidic hydrogen atom. Structurally, carboxylic acids and esters are related to one another in the same way as alcohols and ethers.
Ester 
Esters can be formed by heating carboxylic acids and alcohols in the presence of an acid catalyst. This process is reversible, and the starting materials can be regenerated by reacting an ester with water in the presence of a weak base.
Some esters have very pleasant odors, so they are used in the manufacture of many perfumes. Propyl acetate contributes to the odor of pears, while isoamyl acetate gives bananas their smell. This ester also serves as an alarm signal for honeybees. Esters are employed in the manufacture of fabrics (polyesters) and Plexiglass. Anesthetics such as procaine and benzocaine also contain esters.
Amides
An amide is a carbonyl in which the carbon is attached to one nitrogen atom and one carbon or hydrogen atom. Alternatively, we could define an amide as an amine in which one of the carbon atoms attached to the nitrogen is part of a carbonyl.
Amide 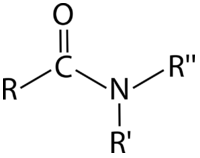
An amide can be formed by combining a carboxylic acid and an amine. Amides are used as coloring agents in crayons, pencils, and ink. They are employed in the paper, plastic, and rubber industries. Polyacrylamide is a very widely used amide; it is involved in t he treatment of drinking water and sewage, and in plastics manufacture. The amide Kevlar is widely employed for the production of body armor, and nylon is another type of amide-based polymer.
Haloalkanes
The haloalkanes, also known as alkyl halides (R–X), are a group of chemical compounds comprised of an alkane with one or more hydrogens replaced by a halogen atom (Group 17 atom). There is a fairly large distinction between the structural and physical properties of haloalkanes and the structural and physical properties of alkanes.
Haloalkanes are found in fire extinguishers, refrigerants, propellants, solvents, and medications. They are also a significant source of pollution and their use has been reduced or eliminated in some products. Chlorofluorocarbons (CFCs) were used as refrigerants in air-conditioners but were found to be a major cause of the depletion of the ozone layer. Research and development of alternatives began in the 1970s. Hydrochlorofluorocarbons (HCFCs) have been used for many years since they cause less damage to the ozone layer, but many countries agreed to eliminate HCFCs by 2020.
Summary
The functional group, a structural arrangement of atoms and/or bonds, is largely responsible for the properties of organic compound families.
Concept Review Exercises
-
What is the functional group of an alkene? An thiol?
-
Does CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3 have a functional group? Explain.
Answers
-
Alkenes contain a carbon-to-carbon double bond (C=C). Thiols have contain a sulfur bonded to hydrogen (SH).
-
No; it has nothing but carbon and hydrogen atoms and all single bonds. (Plain alkanes are often thought to be the absence of a functional group.)
Exercises
- What is the functional group of 1-butanol (CH3CH2CH2CH2OH)?
- What are the functional groups in CH3NHCH2CH2CHO?
- What functional group or groups are present in CH3COOCH2CONHCH3?
- The molecule below contains four different functional groups. Label them.
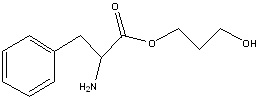
Answer
- OH, an alcohol
- NH, an amine, and CHO, an aldehyde
- COOC, an ester, and CONH, and amide
.jpg?revision=1)