11.5: Alkenes
- Page ID
- 83135
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- To differentiate the structure of alkenes (and other unsaturated hydrocarbons, like alkynes and aromatics) from saturated alkanes.
- Recognize that alkenes that can exist as geometric isomers and classify isomers as cis or trans.
Our modern society is based to a large degree on the chemicals we discuss in this chapter. Most are made from petroleum. Alkanes—saturated hydrocarbons—have relatively few important chemical properties other than that they undergo combustion and react with halogens. Unsaturated hydrocarbons—hydrocarbons with double or triple bonds—on the other hand, are quite reactive. In fact, they serve as building blocks for many familiar plastics—polyethylene, vinyl plastics, acrylics—and other important synthetic materials (e.g., alcohols, antifreeze, and detergents). Aromatic hydrocarbons have formulas that can be drawn as cyclic alkenes, making them appear unsaturated, but their structure and properties are generally quite different, so they are not considered to be alkenes. Aromatic compounds serve as the basis for many drugs, antiseptics, explosives, solvents, and plastics (e.g., polyesters and polystyrene). The two simplest unsaturated compounds—ethylene (ethene) and acetylene (ethyne)—were once used as anesthetics and were introduced to the medical field in 1924. However, it was discovered that acetylene forms explosive mixtures with air, so its medical use was abandoned in 1925. Ethylene was thought to be safer, but it too was implicated in numerous lethal fires and explosions during anesthesia. Even so, it remained an important anesthetic into the 1960s, when it was replaced by nonflammable anesthetics such as halothane (CHBrClCF3CHBrClCF3).
As noted before, alkenes are hydrocarbons with carbon-to-carbon double bonds (R2C=CR2) and alkynes are hydrocarbons with carbon-to-carbon triple bonds (R–C≡C–R). Collectively, they are called unsaturated hydrocarbons because they have fewer hydrogen atoms than does an alkane with the same number of carbon atoms, as is indicated in the following general formulas:
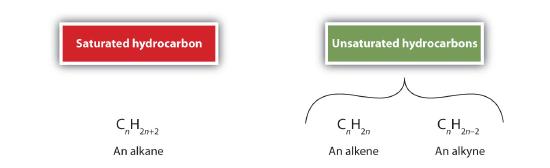
Ethene condensed structural formulas, like H2C=CH2, CH2=CH2, or even CH2CH2 all stand for
The double bond is shared by the two carbons and does not involve the hydrogen atoms, although the condensed formula does not make this point obvious. Note that the molecular formula for ethene is C2H4, whereas that for ethane is C2H6.
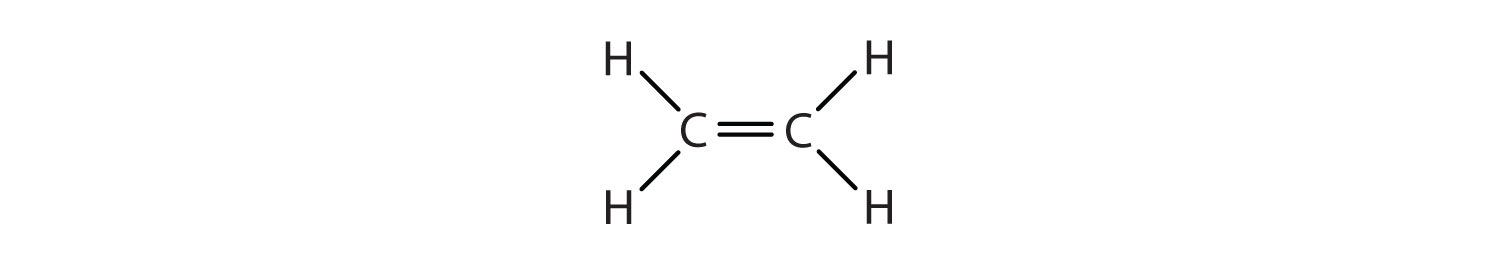
The first two alkenes in Table \(\PageIndex{1}\), ethene and propene, are most often called by their common names—ethylene and propylene, respectively (Figure \(\PageIndex{1}\)). Ethylene is a major commercial chemical. The US chemical industry produces about 25 billion kilograms of ethylene annually, more than any other synthetic organic chemical. More than half of this ethylene goes into the manufacture of polyethylene, one of the most familiar plastics. Propylene is also an important industrial chemical. It is converted to plastics, isopropyl alcohol, and a variety of other products.
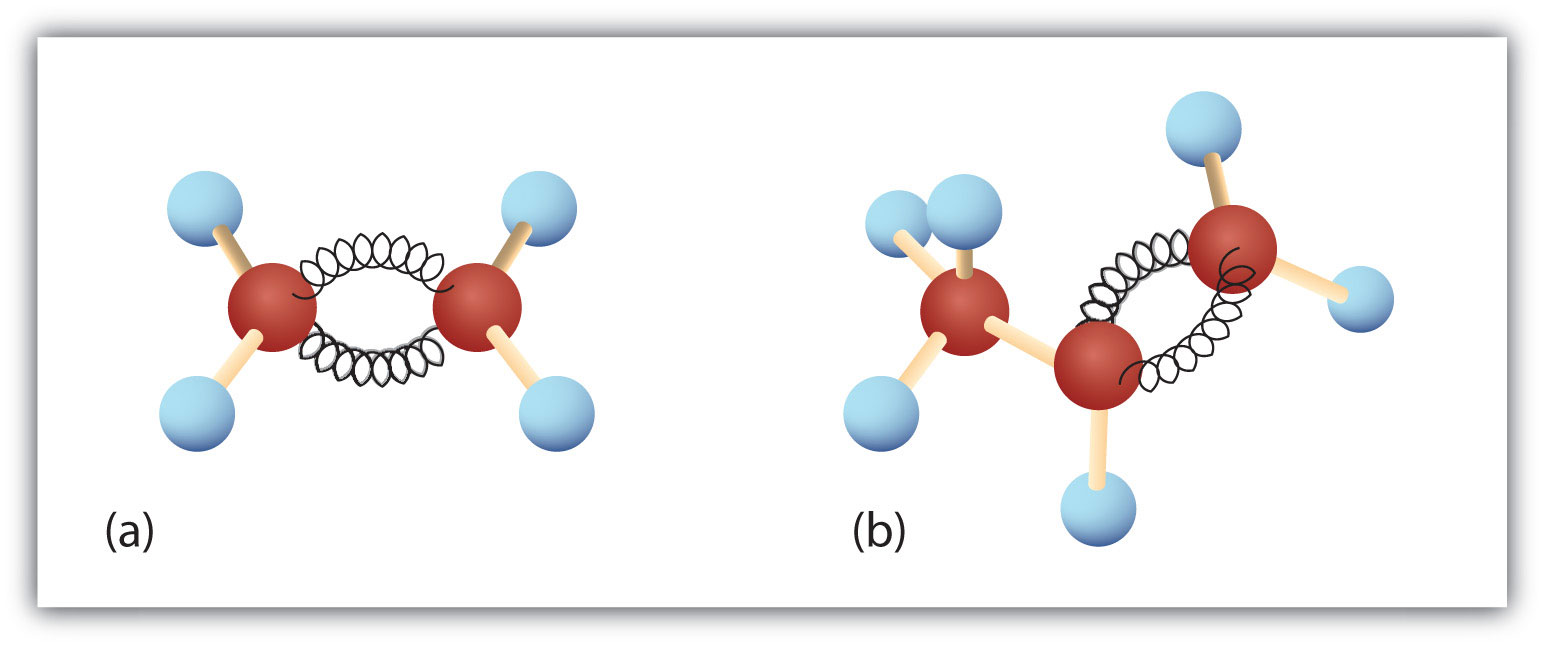
Figure \(\PageIndex{1}\): Ethene and Propene. The ball-and-spring models of ethene/ethylene (a) and propene/propylene (b) show their respective shapes, especially bond angles.
Although there is only one alkene with the formula C2H4 (ethene) and only one with the formula C3H6 (propene), there are several alkenes with the formula C4H8.
Cis-Trans Geometric Isomers
There is free rotation about the carbon-to-carbon single bonds (C–C) in alkanes. In contrast, the structure of alkenes requires that the carbon atoms of a double bond and the two atoms bonded to each carbon atom all lie in a single plane, and that each doubly bonded carbon atom lies in the center of a triangle. This part of the molecule’s structure is rigid; rotation about doubly bonded carbon atoms is not possible without rupturing the bond. Look at the two chlorinated hydrocarbons in Figure \(\PageIndex{2}\).
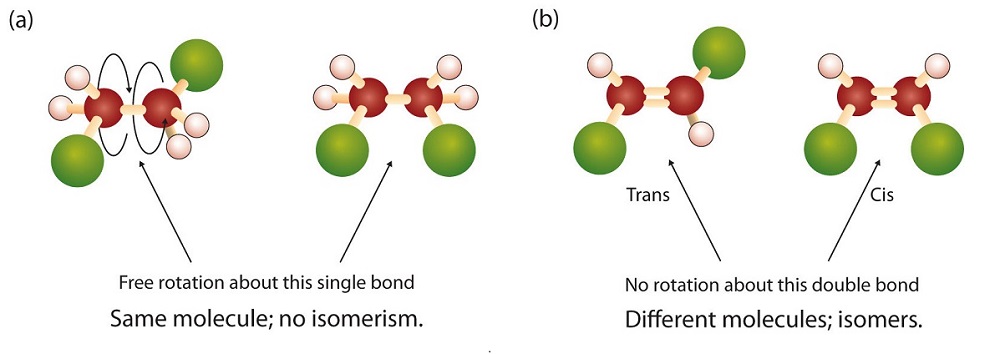
Figure \(\PageIndex{2}\): Rotation about Bonds. In 1,2-dichloroethane (a), free rotation about the C–C bond allows the two structures to be interconverted by a twist of one end relative to the other. In 1,2-dichloroethene (b), restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond are significant.
In 1,2-dichloroethane (part (a) of Figure \(\PageIndex{2}\)), there is free rotation about the C–C bond. The two models shown represent exactly the same molecule; they are not isomers. You can draw structural formulas that look different, but if you bear in mind the possibility of this free rotation about single bonds, you should recognize that these two structures represent the same molecule:

In 1,2-dichloroethene (Figure \(\PageIndex{sb}\)), however, restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond become significant. This leads to a special kind of isomerism. The isomer in which the two chlorine (Cl) atoms lie on the same side of the molecule is called the cis isomer (Latin cis, meaning “on this side”) and is named cis-1,2-dichloroethene. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer (Latin trans, meaning “across”) and is named trans-1,2-dichloroethene. These two compounds are cis-trans isomers (or geometric isomers), compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule.
Consider the alkene with the condensed structural formula CH3CH=CHCH3. We could name it 2-butene, but there are actually two such compounds; the double bond results in cis-trans isomerism (Figure \(\PageIndex{3}\)).
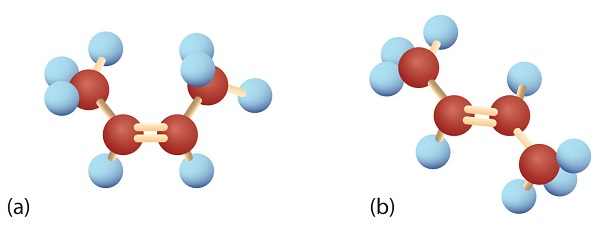
Figure \(\PageIndex{3}\): Ball-and-Spring Models of (a) Cis-2-Butene and (b) Trans-2-Butene. Cis-trans isomers have different physical, chemical, and physiological properties.
Cis-2-butene has both methyl groups on the same side of the molecule. Trans-2-butene has the methyl groups on opposite sides of the molecule. Their structural formulas are as follows:

Note, however, that the presence of a double bond does not necessarily lead to cis-trans isomerism (Figure \(\PageIndex{5}\)). We can draw two seemingly different propenes:

However, these two structures are not really different from each other. If you could pick up either molecule from the page and flip it over top to bottom, you would see that the two formulas are identical. Thus there are two requirements for cis-trans isomerism:
- Rotation must be restricted in the molecule.
- There must be two nonidentical groups on each doubly bonded carbon atom.
In these propene structures, the second requirement for cis-trans isomerism is not fulfilled. One of the doubly bonded carbon atoms does have two different groups attached, but the rules require that both carbon atoms have two different groups. In general, the following statements hold true in cis-trans isomerism:
- Alkenes with a C=CH2 unit do not exist as cis-trans isomers.
- Alkenes with a C=CR2 unit, where the two R groups are the same, do not exist as cis-trans isomers.
- Alkenes of the type R–CH=CH–R can exist as cis and trans isomers; cis if the two R groups are on the same side of the carbon-to-carbon double bond, and trans if the two R groups are on opposite sides of the carbon-to-carbon double bond.
Advanced Note: E/Z Isomerization
If a molecule has a C=C bond with one non-hydrogen group attached to each of the carbons, cis/trans nomenclature descried above is enough to describe it. However, if you have three different groups (or four), then the cis/trans approach is insufficient to describe the different isomers, since we do not know which two of the three groups are being described. For example, if you have a C=C bond, with a methyl group and a bromine on one carbon , and an ethyl group on the other, it is neither trans nor cis, since it is not clear whether the ethyl group is trans to the bromine or the methyl. This is addressed with a more advanced E/Z nomenclature discussed elsewhere.
Example \(\PageIndex{1}\)
Which compounds can exist as cis-trans (geometric) isomers? Draw them.
- CHCl=CHBr
- CH2=CBrCH3
- (CH3)2C=CHCH2CH3
- CH3CH=CHCH2CH3
SOLUTION
All four structures have a double bond and thus meet rule 1 for cis-trans isomerism.
-
This compound meets rule 2; it has two nonidentical groups on each carbon atom (H and Cl on one and H and Br on the other). It exists as both cis and trans isomers:

- This compound has two hydrogen atoms on one of its doubly bonded carbon atoms; it fails rule 2 and does not exist as cis and trans isomers.
- This compound has two methyl (CH3) groups on one of its doubly bonded carbon atoms. It fails rule 2 and does not exist as cis and trans isomers.
-
This compound meets rule 2; it has two nonidentical groups on each carbon atom and exists as both cis and trans isomers:

Aromatic Compounds
Rings of alternating double and single bonds comprise a distinct class, called aromatic hydrocarbons, with unique structures and properties different than regular alkenes. The simplest of these compounds, benzene (C6H6), is of great commercial importance, but it also has noteworthy health effects.
Benzene is a liquid that smells like gasoline, boils at 80°C, and freezes at 5.5°C. It is the aromatic hydrocarbon produced in the largest volume. It was formerly used to decaffeinate coffee and was a significant component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. It was removed from many product formulations in the 1950s, but others continued to use benzene in products until the 1970s when it was associated with leukemia deaths. Benzene is still important in industry as a precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent for such things as cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited.
To explain the surprising properties of benzene, chemists suppose the molecule has a cyclic, hexagonal, planar structure of six carbon atoms with one hydrogen atom bonded to each. We can write a structure with alternate single and double bonds, either as a full structural formula or as a line-angle formula:
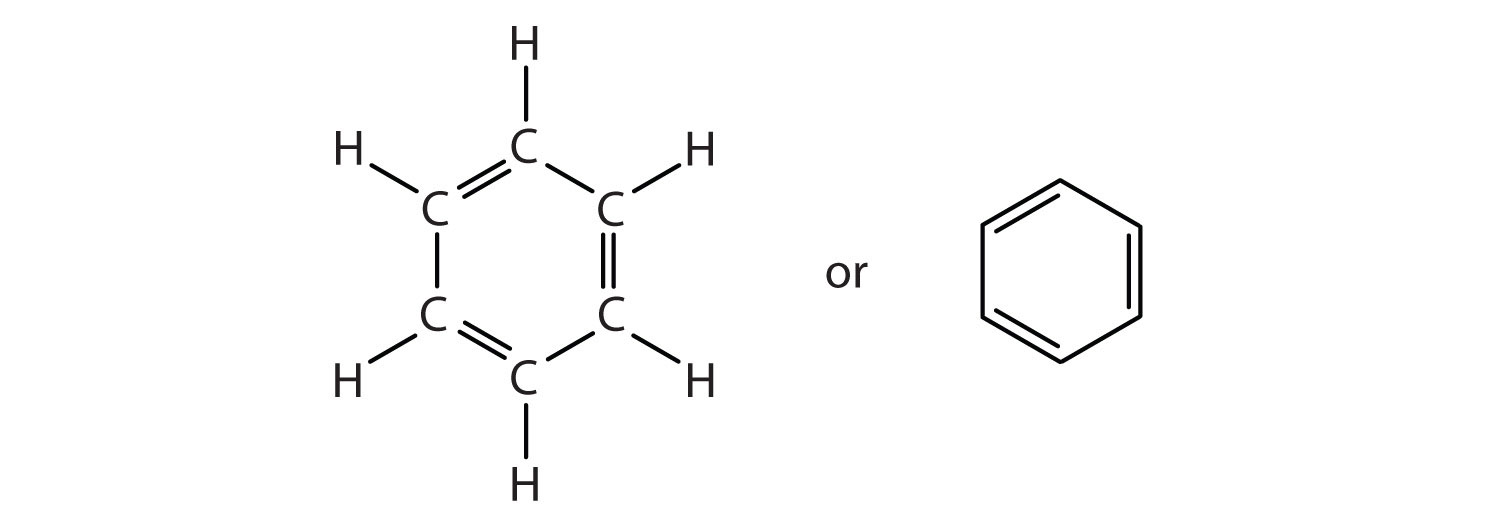
However, these structures do not explain the unique properties of benzene. Furthermore, experimental evidence indicates that all the carbon-to-carbon bonds in benzene are equivalent, and the molecule is unusually stable. Chemists often represent benzene as a hexagon with an inscribed circle:
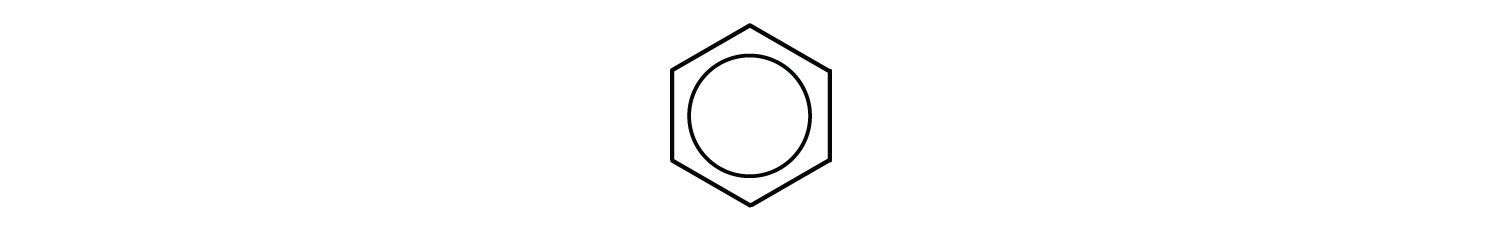
The inner circle indicates that the valence electrons are shared equally by all six carbon atoms (that is, the electrons are delocalized, or spread out, over all the carbon atoms). It is understood that each corner of the hexagon is occupied by one carbon atom, and each carbon atom has one hydrogen atom attached to it. Any other atom or groups of atoms substituted for a hydrogen atom must be shown bonded to a particular corner of the hexagon. We use this modern symbolism, but many scientists still use the earlier structure with alternate double and single bonds.
To Your Health: Benzene and Us
Most of the benzene used commercially comes from petroleum. It is employed as a starting material for the production of detergents, drugs, dyes, insecticides, and plastics. Once widely used as an organic solvent, benzene is now known to have both short- and long-term toxic effects. The inhalation of large concentrations can cause nausea and even death due to respiratory or heart failure, while repeated exposure leads to a progressive disease in which the ability of the bone marrow to make new blood cells is eventually destroyed. This results in a condition called aplastic anemia, in which there is a decrease in the numbers of both the red and white blood cells.
Biologically Important Compounds with Aromatic Rings
Substances containing the benzene ring are common in both animals and plants, although they are more abundant in the latter. Plants can synthesize the benzene ring from carbon dioxide, water, and inorganic materials. Animals cannot synthesize it, but they are dependent on certain aromatic compounds for survival and therefore must obtain them from food. Phenylalanine, tyrosine, and tryptophan (essential amino acids) and vitamins K, B2 (riboflavin), and B9 (folic acid) all contain the benzene ring. Many important drugs, a few of which are shown in Table \(\PageIndex{2}\), also feature a benzene ring.
So far we have examine only aromatic compounds with carbon-containing rings. However, many cyclic compounds have other elements, like oxygen or nitrogen, in the ring. These compounds, called heterocyclic compounds, will be seen in the biochemistry chapters. For example, diazepam (Valium) below has nitrogen atoms as part of the aromatic ring system.
| Name | Structure |
|---|---|
| aspirin |  |
| acetaminophen | 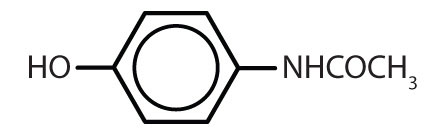 |
| ibuprofen | 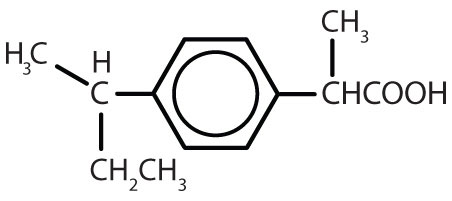 |
| diazepam (Valium) |  |
| sulfanilamide | 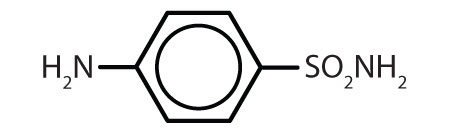 |
Concept Review Exercises
-
Briefly identify the important distinctions between a saturated hydrocarbon and an unsaturated hydrocarbon.
-
Briefly identify the important distinctions between an alkene and an alkane.
-
What are cis-trans (geometric) isomers? What two types of compounds can exhibit cis-trans isomerism?
Answers
- Unsaturated hydrocarbons have double or triple bonds and are quite reactive; saturated hydrocarbons have only single bonds and are rather unreactive.
- An alkene has a double bond; an alkane has single bonds only.
- Cis-trans isomers are compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule. Alkenes and cyclic compounds can exhibit cis-trans isomerism. The alkene must have two non-H groups, one on each double-bond carbon. The cylic compound must have two non-H groups, each on different carbons of ring.
Key Takeaway
- Alkenes are hydrocarbons with a carbon-to-carbon double bond.
- Cis-trans (geometric) isomerism exists when there is restricted rotation in a molecule and there are two nonidentical groups on each doubly bonded carbon atom.
Exercises
1. Classify each compound as saturated or unsaturated. Identify each as an alkane, alkene, alkyne, or aromatic.
-

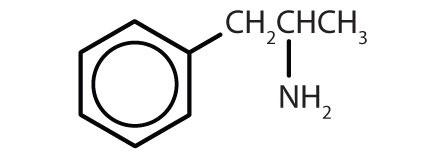
- CH3CH2C≡CCH3
-

2. Classify each compound as a cis isomer, a trans isomer, or neither.
.jpg?revision=1&size=bestfit&width=600)
Answers
-
a. saturated; alkane b. unsaturated; aromatic c. unsaturated; alkyne d. unsaturated; alkene
a. neither (fliping the bond does not change the molecule. There are no isomers for this molecule)
b. trans (the two hydrogen atoms are on different sides, as are the two ethyl groups)
c. cis (the two ethyl groups are on the same side, as are the two hydrogens)
d. trans (notice that the single bond off the double bond on the left is going down, but the other is going up)
3. A. trans-2-pentene; B. cis-2-pentene

