2.10: Periodic Trends
- Page ID
- 469207
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how elements are organized into the periodic table.
- Describe how some characteristics of elements relate to their positions on the periodic table.
As previously noted, the periodic table is arranged so that elements with similar chemical behaviors are in the same group. Chemists often make general statements about the properties of the elements in a group using descriptive names with historical origins. For example, the elements of Group 1 are known as the alkali metals, Group 2 are the alkaline earth metals, Group 17 are the halogens, and Group 18 are the noble gases.
Group 1: The Alkali Metals
The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium. Hydrogen is unique in that it is generally placed in Group 1, but it is not a metal. The compounds of the alkali metals are common in nature and daily life. One example is table salt (sodium chloride); lithium compounds are used in greases, in batteries, and as drugs to treat patients who exhibit manic-depressive, or bipolar, behavior. Although lithium, rubidium, and cesium are relatively rare in nature, and francium is so unstable and highly radioactive that it exists in only trace amounts, sodium and potassium are the seventh and eighth most abundant elements in Earth’s crust, respectively.
Group 2: The Alkaline Earth Metals
The alkaline earth metals are beryllium, magnesium, calcium, strontium, barium, and radium. Beryllium, strontium, and barium are rare, and radium is unstable and highly radioactive. In contrast, calcium and magnesium are the fifth and sixth most abundant elements on Earth, respectively; they are found in huge deposits of limestone and other minerals.
Group 17: The Halogens
The halogens are fluorine, chlorine, bromine, iodine, and astatine. The name halogen is derived from the Greek words for “salt forming,” which reflects that all the halogens react readily with metals to form compounds, such as sodium chloride and calcium chloride (used in some areas as road salt).
Compounds that contain the fluoride ion are added to toothpaste and the water supply to prevent dental cavities. Fluorine is also found in Teflon coatings on kitchen utensils. Although chlorofluorocarbon propellants and refrigerants are believed to lead to the depletion of Earth’s ozone layer and contain both fluorine and chlorine, the latter is responsible for the adverse effect on the ozone layer. Bromine and iodine are less abundant than chlorine, and astatine is so radioactive that it exists in only negligible amounts in nature.
Group 18: The Noble Gases
The noble gases are helium, neon, argon, krypton, xenon, and radon. Because the noble gases are composed of only single atoms, they are called monatomic. At room temperature and pressure, they are unreactive gases. Because of their lack of reactivity, for many years they were called inert gases or rare gases. However, the first chemical compounds containing the noble gases were prepared in 1962. Although the noble gases are relatively minor constituents of the atmosphere, natural gas contains substantial amounts of helium. Because of its low reactivity, argon is often used as an unreactive (inert) atmosphere for welding and in light bulbs. The red light emitted by neon in a gas discharge tube is used in neon lights.
Radon is an invisible, odorless noble gas that is slowly released from the ground, particularly from rocks and soils whose uranium content is high. Because it is a noble gas, radon is not chemically reactive. Unfortunately, it is radioactive, and increased exposure to it has been correlated with an increased lung cancer risk.
Because radon comes from the ground, we cannot avoid it entirely. Moreover, because it is denser than air, radon tends to accumulate in basements, which if improperly ventilated can be hazardous to a building’s inhabitants. Fortunately, specialized ventilation minimizes the amount of radon that might collect. Special fan-and-vent systems are available that draw air from below the basement floor, before it can enter the living space, and vent it above the roof of a house.
After smoking, radon is thought to be the second-biggest preventable cause of lung cancer in the United States. The American Cancer Society estimates that 10% of all lung cancers are related to radon exposure. There is uncertainty regarding what levels of exposure cause cancer, as well as what the exact causal agent might be (either radon or one of its breakdown products, many of which are also radioactive and, unlike radon, not gases). The US Environmental Protection Agency recommends testing every floor below the third floor for radon levels to guard against long-term health effects.
Why do elements in a given group have similar properties?
The periodic table is organized on the basis of similarities in elemental properties, but what explains these similarities? It turns out that the shape of the periodic table reflects the filling of subshells with electrons, as shown in Figure \(\PageIndex{4}\). Starting with the first period and going from left to right, the table reproduces the order of filling of the electron subshells in atoms. Furthermore, elements in the same group share the same valence shell electron configuration. For example, all elements in the first column have a single s electron in their valence shells, so their electron configurations can be described as ns1 (where n represents the shell number). This last observation is crucial. Chemistry is largely the result of interactions between the valence electrons of different atoms. Thus, atoms that have the same valence shell electron configuration will have similar chemistry.

Using the variable n to represent the number of the valence electron shell, write the valence shell electron configuration for each group.
- the alkaline earth metals
- the column of elements headed by carbon
- Answer a
-
The alkaline earth metals are in the second column of the periodic table. This column corresponds to the s subshell being filled with 2 electrons. Therefore, the valence shell electron configuration is ns2.
- Answer b
-
The electron configuration of carbon is 1s22s22p2. Its valence shell electron configuration is 2s22p2. Every element in the same column should have a similar valence shell electron configuration, which we can represent as ns2np2.
Using the variable n to represent the number of the valence electron shell, write the valence shell electron configuration for each group.
- the halogens
- the column of elements headed by oxygen
- Answer a
-
The halogens are in the 17th column (or Group 7A) of the periodic table. This column corresponds to the p subshell being filled with 5 electrons. Therefore, the valence shell electron configuration is ns2np5.
- Answer b
-
The column headed by O is the 16th column (or Group 6A). This column corresponds to the p subshell being filled with 4 electrons. Therefore, the valence shell electron configuration is ns2np4.
Valence Electrons and Group Number
The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column.
| Periodic table group | Valence electrons |
|---|---|
| Group 1 (I) (alkali metals) | 1 |
| Group 2 (II) (alkaline earth metals) | 2 |
| Groups 3-12 (transition metals) | 2* |
| Group 13 (III) (boron group) | 3 |
| Group 14 (IV) (carbon group) | 4 |
| Group 15 (V) (pnictogens) | 5 |
| Group 16 (VI) (chalcogens) | 6 |
| Group 17 (VII) (halogens) | 7 |
| Group 18 (VIII or 0) (noble gases) | 8** |
* The general method for counting valence electrons is generally not useful for transition metals.
** Except for helium, which has only two valence electrons.
Atomic Radius
The periodic table is useful for understanding atomic properties that show periodic trends. One such property is the atomic radius (Figure \(\PageIndex{5}\)). The atomic radius is defined as one-half the distance between the nuclei of identical atoms that are bonded together. The units for atomic radii are picometers, equal to \(10^{-12}\) meters. As an example, the internuclear distance between the two hydrogen atoms in an \(\ce{H_2}\) molecule is measured to be \(74 \: \text{pm}\). Therefore, the atomic radius of a hydrogen atom is \(\frac{74}{2} = 37 \: \text{pm}\).
As mentioned earlier, the higher the shell number, the farther from the nucleus the electrons in that shell are likely to be. In other words, the size of an atom is generally determined by the number of the valence electron shell. Therefore, as we go down a column on the periodic table, the atomic radius increases. As we go across a period on the periodic table, however, electrons are being added to the same valence shell; meanwhile, more protons are being added to the nucleus, so the positive charge of the nucleus is increasing. The increasing positive charge attracts the electrons more strongly, pulling them closer to the nucleus. Consequently, as we go across a period, from left to right, the atomic radius decreases. These trends are seen clearly in Figure \(\PageIndex{5}\)
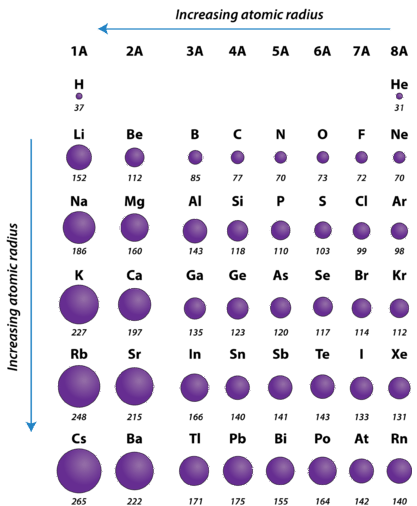
Using the periodic table (rather than Figure \(\PageIndex{5}\)), which atom is larger?
- N or Bi
- Mg or Cl
- Answer a
-
Bi is below N in Group 5A in the periodic table and has electrons in higher-numbered shells, hence we expect that Bi atoms are larger than N atoms.
- Answer b
-
Both Mg and Cl are in period 3 of the periodic table, but Cl lies farther to the right. Therefore we expect Mg atoms to be larger than Cl atoms.
Using the periodic table (rather than Figure \(\PageIndex{5}\)), which atom is larger?
- Li or F
- Na or K
- Answer a
-
Li and F are on the same period, but F lies farther to the right. Therefore, we expect Li to be larger than F atoms.
- Answer b
-
K lies below Na in Group 1A, hence has more electron shells, making it larger than Na.
Clinical chemistry is the area of chemistry concerned with the analysis of body fluids to determine the health status of the human body. Clinical chemists measure a variety of substances, ranging from simple elements such as sodium and potassium to complex molecules such as proteins and enzymes, in blood, urine, and other body fluids. The absence or presence, or abnormally low or high amounts, of a substance can be a sign of some disease or an indication of health. Many clinical chemists use sophisticated equipment and complex chemical reactions in their work, so they not only need to understand basic chemistry, but also be familiar with special instrumentation and how to interpret test results.
Concept Review Exercises
- How are the elements organized into the periodic table?
- Looking at the periodic table, where do the following elements appear?
- the metals
- the nonmetals
- the halogens
- the transition metals
- the noble gases
- Describe the trends in atomic radii as related to an element’s position on the periodic table.
Answers
- Elements are organized in order of increasing atomic number.
-
- the left three-quarters of the periodic table (to the left of the zigzag band)
- the right quarter of the periodic table (to the right of the zigzag band)
- the next-to-last column of the periodic table
- the middle section of the periodic table
- the last column of the periodic table
- As you go across the periodic table, atomic radii decrease; as you go down the periodic table, atomic radii increase.
Key Takeaways
- The chemical elements are arranged in a chart called the periodic table.
- Some characteristics of the elements are related to their position on the periodic table.
- The number of valence electrons of an element can be determined by the group (vertical column) number in the Periodic Table. Elements with the same number of valence electrons have similar chemical properties.


