Cycloalkanes are cyclic hydrocarbons, meaning that the carbons of the molecule are arranged in the form of a ring. Cycloalkanes are also saturated, meaning that all of the carbons atoms that make up the ring are single bonded to other atoms (no double or triple bonds). There are also polycyclic alkanes, which are molecules that contain two or more cycloalkanes that are joined, forming multiple rings.
Introduction
Many organic compounds found in nature or created in a laboratory contain rings of carbon atoms with distinguishing chemical properties; these compounds are known as cycloalkanes. Cycloalkanes only contain carbon-hydrogen bonds and carbon-carbon single bonds, but in cycloalkanes, the carbon atoms are joined in a ring. The smallest cycloalkane is cyclopropane.
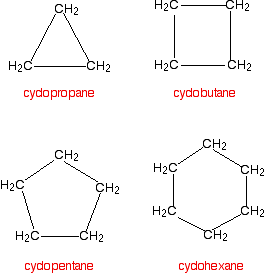
Figure 1: The first four cycloalkanes
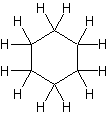
If you count the carbons and hydrogens, you will see that they no longer fit the general formula \(C_nH_{2n+2}\). By joining the carbon atoms in a ring,two hydrogen atoms have been lost. The general formula for a cycloalkane is \(C_nH_{2n}\). Cyclic compounds are not all flat molecules. All of the cycloalkanes, from cyclopentane upwards, exist as "puckered rings". Cyclohexane, for example, has a ring structure that looks like this:
Figure 2: This is known as the "chair" form of cyclohexane from its shape, which vaguely resembles a chair. Note: The cyclohexane molecule is constantly changing, with the atom on the left, which is currently pointing down, flipping up, and the atom on the right flipping down. During this process, another (slightly less stable) form of cyclohexane is formed known as the "boat" form. In this arrangement, both of these atoms are either pointing up or down at the same time
In addition to being saturated cyclic hydrocarbons, cycloalkanes may have multiple substituents or functional groups that further determine their unique chemical properties. The most common and useful cycloalkanes in organic chemistry are cyclopentane and cyclohexane, although other cycloalkanes varying in the number of carbons can be synthesized. Understanding cycloalkanes and their properties are crucial in that many of the biological processes that occur in most living things have cycloalkane-like structures.
Although polycyclic compounds are important, they are highly complex and typically have common names accepted by IUPAC. However, the common names do not generally follow the basic IUPAC nomenclature rules. The general formula of the cycloalkanes is \(C_nH_{2n}\) where \(n\) is the number of carbons. The naming of cycloalkanes follows a simple set of rules that are built upon the same basic steps in naming alkanes. Cyclic hydrocarbons have the prefix "cyclo-".
IUPAC Rules for Nomenclature
- Determine the cycloalkane to use as the parent chain. The parent chain is the one with the highest number of carbon atoms. If there are two cycloalkanes, use the cycloalkane with the higher number of carbons as the parent chain.
- If there is an alkyl straight chain that has a greater number of carbons than the cycloalkane, then the alkyl chain must be used as the primary parent chain. Cycloalkane acting as a substituent to an alkyl chain has an ending "-yl" and, therefore, must be named as a cycloalkyl.
| Cycloalkane |
Cycloalkyl |
| cyclopropane |
cyclopropyl |
| cyclobutane |
cyclobutyl |
| cyclopentane |
cyclopentyl |
| cyclohexane |
cyclohexyl |
| cycloheptane |
cycloheptyl |
| cyclooctane |
cyclooctyl |
| cyclononane |
cyclononanyl |
| cyclodecane |
cyclodecanyl |
Example 1
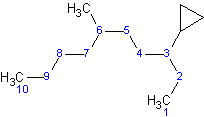
The longest straight chain contains 10 carbons, compared with cyclopropane, which only contains 3 carbons. Because cyclopropane is a substituent, it would be named a cyclopropyl-substituted alkane.
- Determine any functional groups or other alkyl groups.
- Number the carbons of the cycloalkane so that the carbons with functional groups or alkyl groups have the lowest possible number. A carbon with multiple substituents should have a lower number than a carbon with only one substituent or functional group. One way to make sure that the lowest number possible is assigned is to number the carbons so that when the numbers corresponding to the substituents are added, their sum is the lowest possible.
.gif?revision=1&size=bestfit&width=171&height=129) (1+3=4) NOT
(1+3=4) NOT .gif?revision=1&size=bestfit&width=171&height=132) (1+5=6)
(1+5=6)
- When naming the cycloalkane, the substituents and functional groups must be placed in alphabetical order.
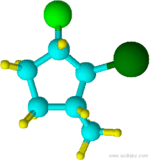
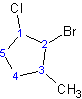
(ex: 2-bromo-1-chloro-3-methylcyclopentane)
- Indicate the carbon number with the functional group with the highest priority according to alphabetical order. A dash"-" must be placed between the numbers and the name of the substituent. After the carbon number and the dash, the name of the substituent can follow. When there is only one substituent on the parent chain, indicating the number of the carbon atoms with the substituent is not necessary.
(ex: 1-chlorocyclohexane or cholorocyclohexane is acceptable) 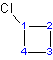
- If there is more than one of the same functional group on one carbon, write the number of the carbon two, three, or four times, depending on how many of the same functional group is present on that carbon. The numbers must be separated by commas, and the name of the functional group that follows must be separated by a dash. When there are two of the same functional group, the name must have the prefix "di". When there are three of the same functional group, the name must have the prefix "tri". When there are four of the same functional group, the name must have the prefix "tetra". However, these prefixes cannot be used when determining the alphabetical priorities. There must always be commas between the numbers and the dashes that are between the numbers and the names.
Example 2
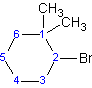
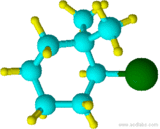
(2-bromo-1,1-dimethylcyclohexane)
Notice that "f" of fluoro alphabetically precedes the "m" of methyl. Although "di" alphabetically precedes "f", it is not used in determining the alphabetical order.
Example 3

(2-fluoro-1,1,-dimethylcyclohexane NOT 1,1-dimethyl-2-fluorocyclohexane)
8) If the substituents of the cycloalkane are related by the cis or trans configuration, then indicate the configuration by placing "cis-" or "trans-" in front of the name of the structure.
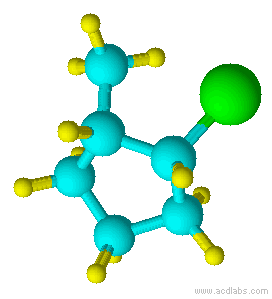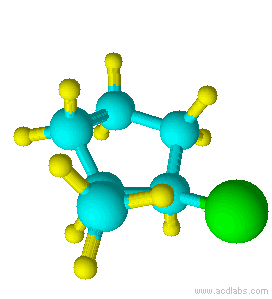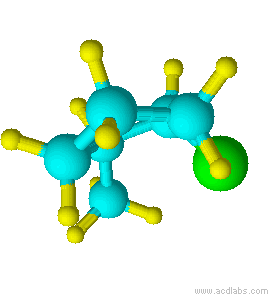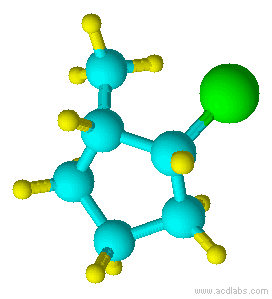
Blue=Carbon Yellow=Hydrogen Green=Chlorine
Notice that chlorine and the methyl group are both pointed in the same direction on the axis of the molecule; therefore, they are cis.
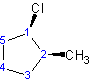 cis-1-chloro-2-methylcyclopentane
cis-1-chloro-2-methylcyclopentane
9) After all the functional groups and substituents have been mentioned with their corresponding numbers, the name of the cycloalkane can follow.
Reactivity
Cycloalkanes are very similar to the alkanes in reactivity, except for the very small ones, especially cyclopropane. Cyclopropane is significantly more reactive than what is expected because of the bond angles in the ring. Normally, when carbon forms four single bonds, the bond angles are approximately 109.5°. In cyclopropane, the bond angles are 60°.
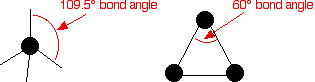
With the electron pairs this close together, there is a significant amount of repulsion between the bonding pairs joining the carbon atoms, making the bonds easier to break.
Alcohol Substituents on Cycloalkanes
Alcohol (-OH) substituents take the highest priority for carbon atom numbering in IUPAC nomenclature. The carbon atom with the alcohol substituent must be labeled as 1. Molecules containing an alcohol group have an ending "-ol", indicating the presence of an alcohol group. If there are two alcohol groups, the molecule will have a "di-" prefix before "-ol" (diol). If there are three alcohol groups, the molecule will have a "tri-" prefix before "-ol" (triol), etc.
Example 4
The alcohol substituent is given the lowest number even though the two methyl groups are on the same carbon atom and labeling 1 on that carbon atom would give the lowest possible numbers. Numbering the location of the alcohol substituent is unnecessary because the ending "-ol" indicates the presence of one alcohol group on carbon atom number 1.
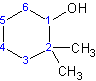
2,2-dimethylcyclohexanol NOT 1,1-dimethyl-cyclohexane-2-ol
Example 5
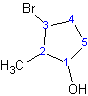
3-bromo-2-methylcyclopentanol NOT 1-bromo-2-methyl-cyclopentane-2-ol
Example 6
.gif?revision=1&size=bestfit&width=173&height=173)
Blue=Carbon Yellow=Hydrogen Red=Oxygen
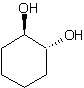 trans-cyclohexane-1,2-diol
trans-cyclohexane-1,2-diol
Other Substituents on Cycloalkanes
There are many other functional groups like alcohol, which are later covered in an organic chemistry course, and they determine the ending name of a molecule. The naming of these functional groups will be explained in depth later as their chemical properties are explained.
| Name |
Name ending |
| alkene |
-ene |
| alkyne |
-yne |
| alcohol |
-ol |
| ether |
-ether |
| nitrile |
-nitrile |
| amine |
-amine |
| aldehyde |
-al |
| ketone |
-one |
| carboxylic acid |
-oic acid |
| ester |
-oate |
| amide |
-amide |
Although alkynes determine the name ending of a molecule, alkyne as a substituent on a cycloalkane is not possible because alkynes are planar and would require that the carbon that is part of the ring form 5 bonds, giving the carbon atom a negative charge.
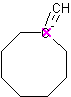
However, a cycloalkane with a triple bond-containing substituent is possible if the triple bond is not directly attached to the ring.
Example 7
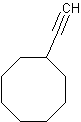
ethynylcyclooctane
Example 8
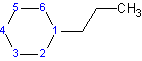
1-propylcyclohexane
Summary
- Determine the parent chain: the parent chain contains the most carbon atoms.
- Number the substituents of the chain so that the sum of the numbers is the lowest possible.
- Name the substituents and place them in alphabetical order.
- If stereochemistry of the compound is shown, indicate the orientation as part of the nomenclature.
- Cyclic hydrocarbons have the prefix "cyclo-" and have an "-alkane" ending unless there is an alcohol substituent present. When an alcohol substituent is present, the molecule has an "-ol" ending.
Glossary
- alcohol: An oxygen and hydrogenOH hydroxyl group that is bonded to a substituted alkyl group.
- alkyl: A structure that is formed when a hydrogen atom is removed from an alkane.
- cyclic: Chemical compounds arranged in the form of a ring or a closed chain form.
- cycloalkanes: Cyclic saturated hydrocarbons with a general formula of CnH(2n). Cycloalkanes are alkanes with carbon atoms attached in the form of a closed ring.
- functional groups: An atom or groups of atoms that substitute for a hydrogen atom in an organic compound, giving the compound unique chemical properties and determining its reactivity.
- hydrocarbon: A chemical compound containing only carbon and hydrogen atoms.
- saturated: All of the atoms that make up a compound are single bonded to the other atoms, with no double or triple bonds.
- skeletal structure: A simplified structure in which each intersection between two lines is assumed to have a carbon atom with its corresponding number of hydrogens.
Problems
Name the following structures. (Note: The structures are complex for practice purposes and may not be found in nature.)
1)  2)
2) 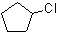 3)
3)  4)
4)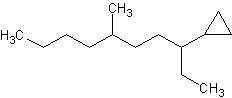 5)
5)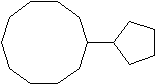 6)
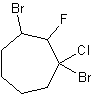
6)
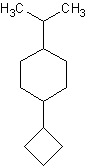
7)
Draw the following structures.
8) 1,1-dibromo-5-fluoro-3-butyl-7-methylcyclooctane 9) trans-1-bromo-2-chlorocyclopentane
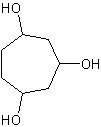
10) 1,1-dibromo-2,3-dichloro-4-propylcyclobutane 11) 2-methyl-1-ethyl-1,3-dipropylcyclopentane 12) cycloheptane-1,3,5-triol
Name the following structures.
Blue=Carbon Yellow=Hydrogen Red=Oxygen Green=Chlorine
13).gif?revision=1&size=bestfit&width=102&height=105) 14)
14)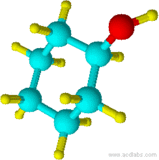 15)
15)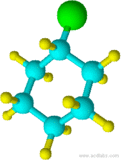 16)
16)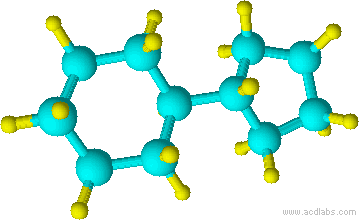 17)
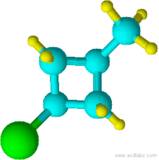
17)
18)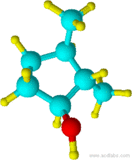 19)
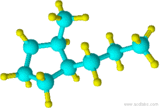
19)
Answers to Practice Problems
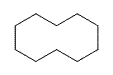
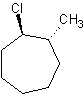
1) cyclodecane 2) chlorocyclopentane or 1-chlorocyclopentane 3) trans-1-chloro-2-methylcycloheptane
4) 3-cyclopropyl-6-methyldecane 5) cyclopentylcyclodecane or 1-cyclopentylcyclodecane 6) 1,3-dibromo-1-chloro-2-fluorocycloheptane
7) 1-cyclobutyl-4-isopropylcyclohexane
8)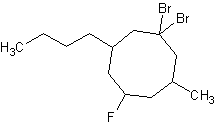 9)
9)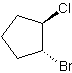 10)
10)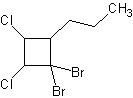 11)
11)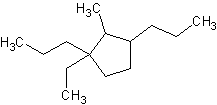 12)
12)
13) cyclohexane 14) cyclohexanol 15) chlorocyclohexane 16) cyclopentylcyclohexane 17) 1-chloro-3-methylcyclobutane
18) 2,3-dimethylcyclohexanol 19) cis-1-propyl-2-methylcyclopentane
References
- ACD/ChemSketch Freeware, version 11.0, Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com, 2008.
- Bruice, Paula Yurkanis. Oragnic Chemistry. 5th. CA. Prentice Hall, 2006.
- Fryhle, C.B. and G. Solomons. Organic Chemistry. 9th ed. Danvers, MA: Wiley, 2008.
- McMurry, John. Organic Chemistry. 7th ed. Belmont, California: Thomson Higher Education, 2008.
- Sadava, Heller, Orians, Purves, Hillis. Life The Science of Biology. 8th ed. Sunderland, MA: W.H. Freeman, 2008.
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry. 5th ed. New York: W.H. Freeman, 2007.
Contributors
- Pwint Zin
- Jim Clark (ChemGuide)





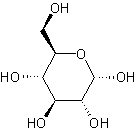
.gif?revision=1&size=bestfit&width=85&height=92)






.gif?revision=1&size=bestfit&width=171&height=129)
.gif?revision=1&size=bestfit&width=171&height=132)











.gif?revision=1&size=bestfit&width=173&height=173)



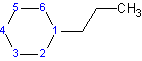
 2)
2) 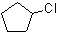 3)
3)  4)
4) 5)
5) 6)
6)

.gif?revision=1&size=bestfit&width=102&height=105)
 15)
15) 16)
16) 17)
17)
 19)
19)
 9)
9) 10)
10) 11)
11) 12)
12)