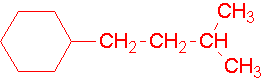2.5: Practice - Alkane Nomenclature
- Page ID
- 205216
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)I. The parent compound must have the longest chain of carbon atoms
Learn: methane (1 carbon atom), ethane (2 carbon atoms), propane (3 carbon atoms), butane (4 carbon atoms), pentane (5 carbon atoms), hexane (6 carbon atoms), heptane (7 carbon atoms), octane (8 carbon atoms), nonane (9 carbon atoms) and decane (10 carbon atoms).
Try to name the following compounds:
- CH3-CH2-CH2-CH3
- CH3-CH2-CH2-CH2-CH2-CH3
- CH3-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH3
Try to draw structures for the following compounds:
- propane
- pentane
- octane
II. The parent chain is numbered to give substituents the lowest possible numbers
Substituent names are methyl, ethyl, propyl, butyl, etc. The number showing the point of attachment to the parent chain precedes the substituent name. If you have more than one substituent with the same name, a number of attachment must be given for each substituent and the number of the substituents must be designated with di-, tri-, etc.
Try to name the following compounds...
7. 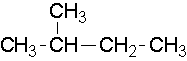
8. 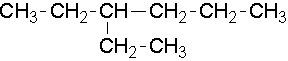
9. 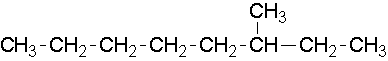
Try to draw structures for the following compounds...
- 2,2-dimethylpropane
- 3-methylheptane
- 4,5-diethylnonane
III. Substituents are named in alphabetical order
Remember that the numbering of the parent chain must give all substituents the lowest possible numbers regardless of their names.
Try to name the following compound...
13. 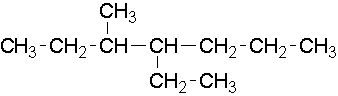
IV. A large substituent is numbered to give its point of attachment
The point of attachment is number one and any other smaller groups are named as substituent groups on the larger group. This numbering is independent of the numbering of the parent chain. Try to name the following compounds...
14.

15. 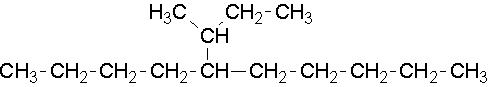
Try to draw structures for the following compounds...
- 4-(1-methylethyl)heptane
- 5-(1,1-dimethylethyl)nonane
V. Ring compounds are designated with a cyclo- prefix and are numbered to give multiple substituents the lowest possible numbers
A single substituent does not need to be numbered. A ring can also be named as a substituent. Try to name the following compounds:
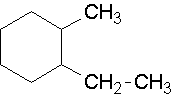
18. 
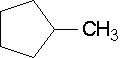
19. 
20. 
Try to draw structures for the following compounds...
- ethylcyclobutane
- 1-ethyl-4-methylcyclohexane
- cyclobutylcyclooctane
VI. Common Names
- isopropyl = 1-methylethyl
- isobutyl = 2-methylpropyl
- sec-butyl = 1-methylpropyl
- tert-butyl = 1,1-dimethylethyl
- neo-pentyl = 2,2-dimethylpropyl
- iso-pentyl = 3-methylbutyl
Try to name the following compounds using common names...
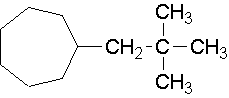
24. 
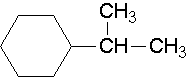
25. 
Try to draw structures for the following compounds...
- 4-isopropyloctane
- isopentylcyclohexane
Answers
- butane
- hexane
- nonane
- CH3-CH2-CH3
- CH3-CH2-CH2-CH2-CH3
- CH3-CH2-CH2-CH2-CH2-CH2-CH2-CH3
- 2-methylbutane
- 3-ethylhexane
- 3-methyloctane (Remember that you must number the parent chain to give the substituent the lowest possible number.)
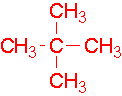

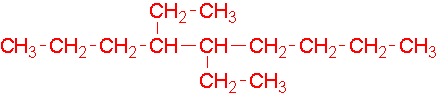
- 4-ethyl-3-methylheptane
-
4-(1-methylethyl)octane
-
5-(1-methylpropyl)decane
-
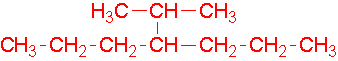
-
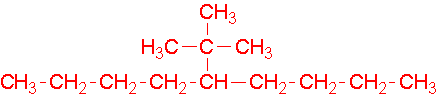
-
1-ethyl-2-methylcyclohexane
-
methylcyclopentane (You do not need a number since there is only one substituent and it is assumed to be on the first carbon regardless of how the ring compound is drawn.)
-
cyclopropylcyclopentane
-
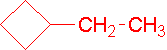
-
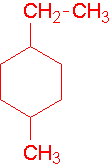
-
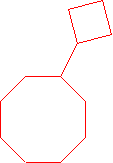
-
neo-pentylcycloheptane (1-(2,2-dimethyl-1-propyl)cycloheptane is the proper name.)
-
isopropylcyclohexane
-
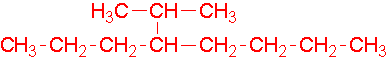
-