11.11: Reaction of Acetylide Anions
- Page ID
- 28240
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation for the reaction that occurs between a terminal alkyne and a strong base, such as sodamide, NaNH2.
- rank a given list of compounds, including water, acetylene and ammonia, in order of increasing or decreasing acidity.
- rank a given list of hydrocarbons, such as acetylene, ethylene and ethane, in order of increasing or decreasing acidity.
- describe a general method for determining which of two given compounds is the stronger acid.
- provide an acceptable explanation of why terminal alkynes are more acidic than alkanes or alkenes.
- write an equation to describe the reaction of an acetylide ion with an alkyl halide.
- discuss the importance of the reaction between acetylide ions and alkyl halides as a method of extending a carbon chain.
- identify the alkyne (and hence the acetylide ion) and the alkyl halide needed to synthesize a given alkyne.
- determine whether or not the reaction of an acetylide ion with a given alkyl halide will result in substitution or elimination, and draw the structure of the product formed in either case.
Make certain that you can define, and use in context, the key terms below.
- acetylide anion
- acidity order
- alkylation
An acetylide anion is an anion formed by removing the proton from the end carbon of a terminal alkyne:
An acidity orderis a list of compounds arranged in order of increasing or decreasing acidity.
The general ideas discussed in this section should already be familiar to you from your previous exposure to chemistry and from the review in Section 2.8. A slightly different account of why terminal alkynes are stronger acids than are alkenes or alkanes is given below. However, the argument is still based on the differences between sp-, sp2- and sp3-hybrid orbitals.
The carbons of a triple bond are sp-hybridized. An sp‑hybrid orbital has a 50% s character and a 50% p character, whereas an sp2‑hybrid orbital is 33% s and 67% p, and an sp3‑hybrid orbital is 25% s and 75% p. The greater the s character of the orbital, the closer the electrons are to the nucleus. Thus in a C(sp)$\ce{-}$H bond, the bonding electrons are closer to the carbon nucleus than they are in a C(sp2)$\ce{-}$H bond. In other words, compared to a C(sp2)$\ce{-}$H bond (or a C(sp3)$\ce{-}$H bond), a C(sp)$\ce{-}$H bond is very slightly polar: Cδ−$\ce{-}$Hδ+. This slight polarity makes it easier for a base to remove a proton from a terminal alkyne than from a less polar or non-polar alkene or alkane.
As you will appreciate, the reaction between sodium amide and a terminal alkyne is an acid-base reaction. The sodium acetylide product is, of course, a salt. Terminal alkynes can also form salts with certain heavy-metal cations, notably silver(I) and copper(I). In the laboratory component of this course, you will use the formation of an insoluble silver acetylide as a method for distinguishing terminal alkynes from alkenes and non-terminal alkynes:
Metal acetylides are explosive when dry. They should be destroyed while still wet by warming with dilute nitric acid:
The alkylation of acetylide ions is important in organic synthesis because it is a reaction in which a new carbon-carbon bond is formed; hence, it can be used when an organic chemist is trying to build a complicated molecule from much simpler starting materials.
The alkyl halide used in this reaction must be primary. Thus, if you were asked for a suitable synthesis of 2,2-dimethyl-3-hexyne, you would choose to attack iodoethane with the anion of 3,3- dimethyl-1-butyne
rather than to attack 2-iodo-2-methylpropane with the anion of 1-butyne.
The reasons will be made clear in Chapter 11.
Acidity of Terminal Alkynes: Formation of Acetylide Anions
Terminal alkynes are much more acidic than most other hydrocarbons. Removal of the terminal proton leads to the formation of an acetylide anion, RC=C:-.
As discussed in Section 2.10, acidity typically increases with the stability of the corresponding conjugate base. The origin of the enhanced acidity of terminal alkynes can be attributed to the stability of the acetylide anion, which has the unpaired electrons in an sp hybridized orbital. The hybridization of an orbital affects its electronegativity. Within a shell, the s orbitals occupy the region closer to the nucleus than the p orbitals. Therefore, the spherical s orbitals are more electronegative than the lobed p orbitals. The relative electronegativity of hybridized orbitals increases as the percent s character increases and follows the order sp > sp2 > sp3. This trend indicates the sp hybridized orbitals of the acetylide anion are more electronegative and better able to stabilize a negative charge than sp2 or sp3 hybridized orbitals. There is a strong correlation between s-character in the orbital containing the non-bonding electrons in the anion and the acidity of hydrocarbons. The table below shows how orbital hybridization compares with the identity of the atom when predicting relative acidity. Remember that as the pKa of a compound decreases its acidity increases.
| Compound | Conjugate Base | Hybridization | "s Character" | pKa | C-H BDE (kJ/mol) |
|---|---|---|---|---|---|
| CH3CH3 | CH3CH2- | sp3 | 25% | 50 | 410 |
| CH2CH2 | CH2CH- | sp2 | 33% | 44 | 473 |
| HCCH | HCC- | sp | 50% | 25 | 523 |
Acetylene, with a pKa of 25 is shown to be much more acidic than ethylene (pKa = 44) or ethane (pKa = 50). Consequently, acetylide anions can be readily formed by deprotonation of a terminal alkynes with a sufficiently strong base. The amide anion (NH2-), in the form of sodium amide (NaNH2) is commonly used for the formation of acetylide anions.
- Given that the pKa of water is 14.00, would you expect hydroxide ion to be capable of removing a proton from each of the substances listed below? Justify your answers, briefly.
- ethanol (pKa = 16)
- acetic acid (pKa = 4.72)
- acetylene (pKa = 25)
- Answer
-
1.
a. No, not very well. The pKa of ethanol is greater than that of water, thus the equilibrium lies to the left rather than to the right.Add texts here. Do not delete this text first.

b. Yes, very well. There is a difference of 11 pKa units between the pKa of water and the pKa of acetic acid. The equilibrium lies well to the right.
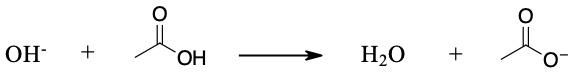
c. No, hardly at all. The hydroxide ion is too weak a base to remove a proton from acetylene. The equilibrium lies well to the left.

Nucleophilic Substitution Reactions of Acetylides
The presence of lone pair electrons and a negative charge on a carbon, makes acetylide anions are strong bases and strong nucleophiles. Therefore, acetylide anions can attack electrophiles such as alkyl halides to cause a substitution reaction. These substitution reactions will be discussed in detail in Chapter 11.
Mechanism
The C-X bonds in 1o alkyl halides are polarized due to the high electronegativity of the halogen. The electrons of the C-X sigma bond are shifted towards the halogen giving it a partial negative charge. This also causes electrons to be shifted away from the carbon giving it a partial positive and making it electrophilic. During this reaction, the lone pair electrons on the acetylide anion attack the electrophilic carbon in the 1o alkyl halide forming a new C-C bond. The formation of this new bond causes the expulsion of the halogen as what is called a leaving group. Overall, this reaction forms a C-C bond and converts a terminal alkyne into a internal alkyne. Because a new alkyl group is added to the alkyne during this reaction, it is commonly called an alkylation.
This substitution reaction is often coupled with the acetylide formation, discussed in the previous section, and shown as a single reaction.

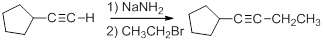
Terminal alkynes can be generated through the reaction of acetylene and a 1o alkyl halide.
Because the acetylide anion is a very strong base, this substitution reaction is most efficient with methyl or primary halides. Secondary, tertiary, or even bulky primary halogens will give alkenes by the E2 elimination mechanism discussed in Section 11.10. An example of this effect is seen in the reaction of bromocyclopentane with a propyne anion. The reaction produces the elimination product cyclopentene rather than the substitution product 1-propynylcyclopentane.
Nucleophilic Addition of Acetylides to Carbonyls
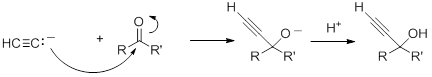
Acetylide anions also add to the electrophilic carbon in aldehydes and ketones to form alkoxides, which, upon protonation, give propargyl alcohols. With aldehydes and non-symmetric ketones, in the absence of chiral catalyst, the product will be a racemic mixture of the two enantiomers. These types of reaction will be discussed in more detail in Chapter 19.
1) The pKa of ammonia is 35. Estimate the equilibrium constant for the deprotonation of pent-1-yne by sodium amide, as shown below.
2) Give the possible reactants which could form the following molecules by an alkylation.
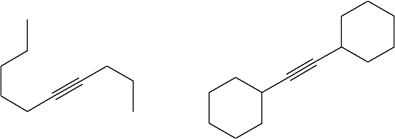
4) Using acetylene as the starting material, show how you would synthesize the following compounds
a)
b) but-2-yne
c)
d)
5) Show how you would accomplish the following synthetic transformation.
- Answer
-
1) Assuming the pKa of pent-1-yne is about 25, then the difference in pKas is 10. Since pentyne is more acidic, the formation of the acetylide will be favored at equilibrium, so the equilibrium constant for the reaction is about 1010.
2)
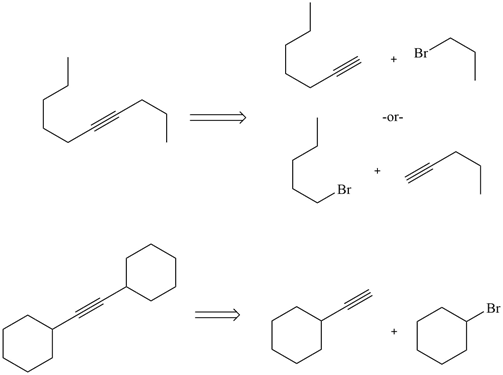
3)

4)
a)
b)
c)
d)
5)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Layne A. Morsch (University of Illinois Springfield)


