Solution Set 10
- Page ID
- 36888
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q1
The molecular orbital diagram for the carbide ion (\(C_2^{2-}\)) would show which of the following molecular orbitals?
- \(\sigma_{1s}^2 {\sigma^*_{1s}}^2 \sigma_{2s}^2 {\sigma*_{2s}}^2 \pi_{2pp}^4\)
- \(\sigma_{1s}^2 {\sigma^*_{1s}}^2 \sigma_{2s}^2 {\sigma^*_{2s}}^2 \pi_{2pp}^4 \sigma_{2p}^2\)
- \(\sigma_{1s}^2 {\sigma^*_{1s}}^2 \sigma_{2s}^2 {\sigma^*_{2s}}^2 \pi_{2pp}^4 \sigma_{2p}^2 {\pi^*_{2p}}^2\)
- \(\sigma_{1s}^2 {\sigma^*_{1s}}^2 \sigma_{2s}^2 {\sigma^*_{2s}}^2 \pi_{2pp}^4 \sigma_{2p}^2 {\pi^*_{2p}}^4\)
A: b
Q2
What is the hybridization of the sulfur atom is \(SF_4\)?
- \(sp^2\)
- \(sp^3\)
- \(sp^3d\)
- \(sp^3d^2\)
A. c
Q3
What is the hybridization of the oxygen atom in water?
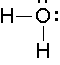
A. \(sp^3\)
Q4
When a double bond is formed between two atoms, one of the bonds is a sigma bond and the other is a pi bond. The pi bond is created by the overlap of...
A. unhybridized p orbitals
Q5
The valence bond hybrid atomic orbitals \(sp^3\) are used by both \(C\) in \(CH_4\) and \(O\) in \(H_2O\). Yet, the bond angles between atoms in \(H_2O\) are less than in \(CH_4\). Explain.
Q6
Describe completely the main features of each of the following and explain what useful information we gain from each.
- Lewis Structures
- Valence Shell Electron Pair Repulsion (VSEPR) theory
- Valence Bond (VB) theory
- Molecular Orbital (MO) theory
Q7
- Draw all possible resonance Lewis structures for \(NO_3^{-1}\). Include formal charges and the correct angles.
- Draw the "realistic" hybrid resonance structure with appropriate angles that takes and average of the Lewis structures in part a. Include formal charges (fractions) and bond orders (fractions). Include nonbonding electrons on central atom but not on terminal atoms.
- Sketch the valence bond probability picture of one of the \(NO_3^{-1}\) resonances. Identify and label the hybridized orbitals. Identify \(\sigma\) and \(\pi\) bonds.
Q8
For each of the following: \(B_2\), \(Ne_2\), \(O_2\)
- Give the molecular orbital (MO) energy diagram for each.
- Write the MO configurations for \(O_2\) starting with (\(\sigma_{1s})^2\)
- Give the bond order of each molecule
- List the species in decreasing order of bond energy and stability
- Identify each as diamagnetic or paramagnetic?
- Using the bond order information, which is least expected to exist. Explain why.
- Which would have the shortest bond length? Explain
Q9
Consider (2E)-3-Methyl-2-nonen-7-yne, an organic molecule with the molecule skeleton structure below:
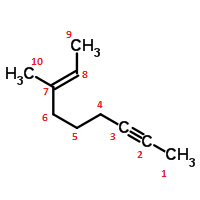
- Draw electron-dot structure for this molecule
- Estimates of bond angles (all of them)
- Identify the hybridization of all heavy (non-H) atoms
- Do all the heavy atoms lie in the same plane?
- What is the description of the bonds formed between the heavy atoms (take into account nature of bond and the hybridization of bonding orbitals). For example: The (C5,C6) single bond is a sigma type bond represented by the overlap of a Carbon sp3 hybridized orbital on C5 with a Carbon sp3 hybridized orbital on C6.
Q10
Several isomers \(CH_2CHNH_2\) are possible. Consider two isomers: ethanimine (on left) and vinylamine (on right)
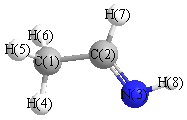
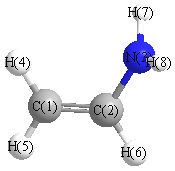
- Draw an acceptable Lewis structure of each isomers.
- Predict the bond angles of this isomer and give the hybridization of each of the heavy (non-H) atoms.
- Describe the bonds formed between the heavy atoms. Use the \(\sigma\) and \(\pi\) formalism in your description.
- Compare the carbon-nitrogen bond lengths. Which is longer and why?
a. ethanimine (on left) and vinylamine (on right)
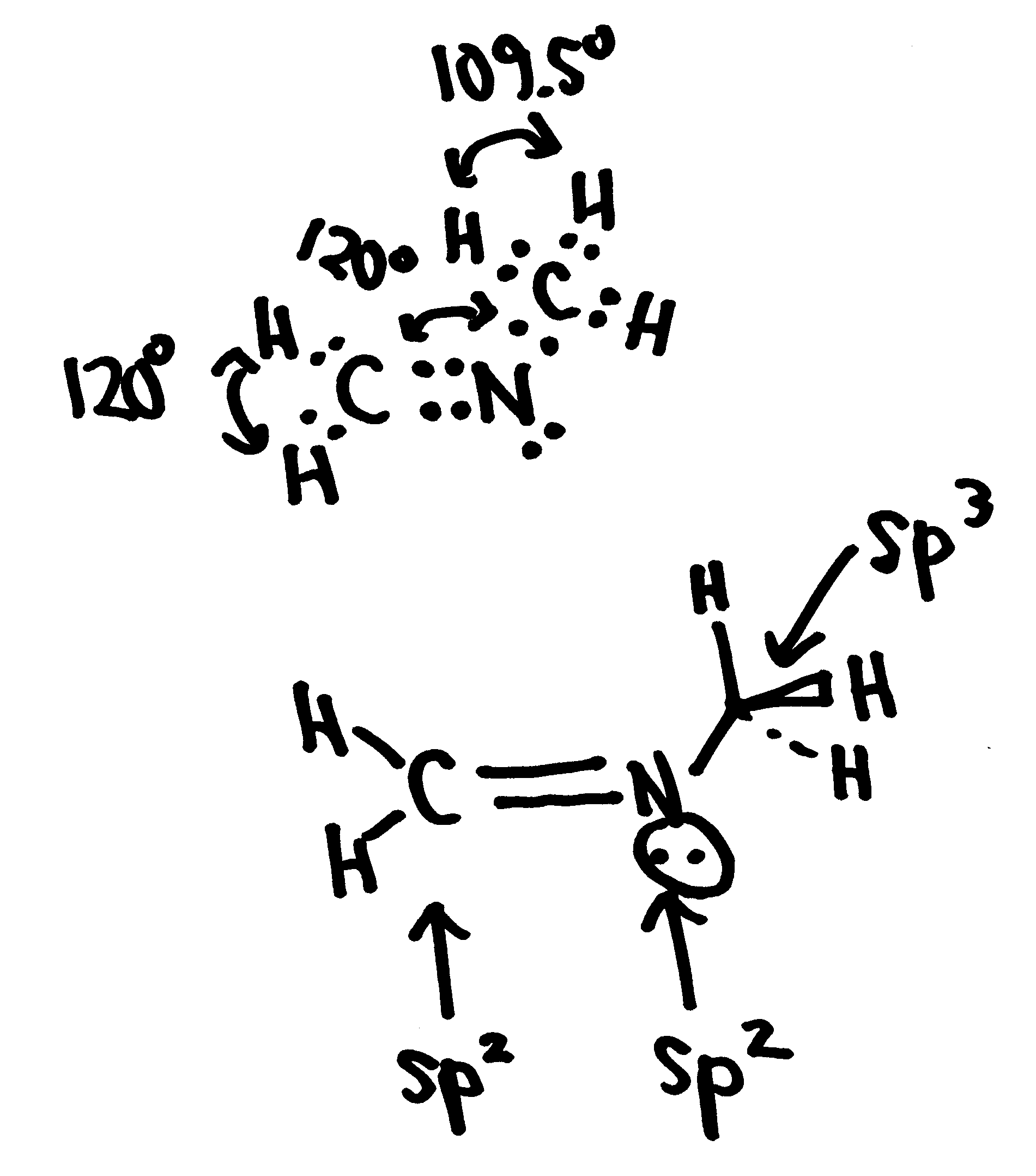
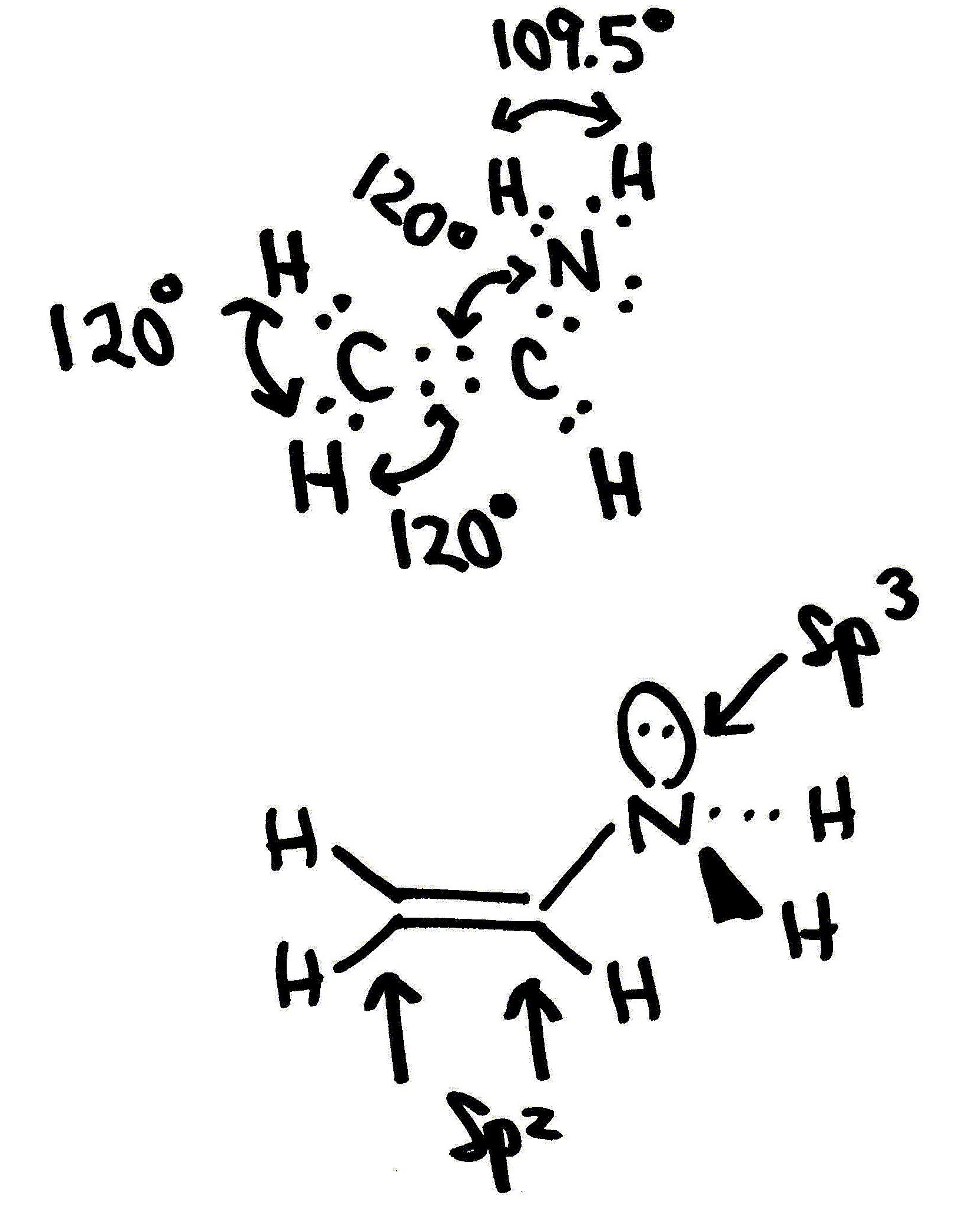
b. Hybridization of the heavy atoms:
- isomer I: both carbons are sp2 hybridized and the nitrogen is sp3 hybridized.
- isomer III: the methyl carbon is sp3 hybridized and the other carbon and the nitrogen in the middle are sp2 hybirdized.
c Describe the bonds formed between the heavy atoms. Use the \(\sigma\) and \(\pi\) formalism in your description.
- isomer I: A double bond is formed between the two carbon atoms that consists of a s-type overlap of two sp2 hybridized orbitals on the two carbons and a p-type overlap of two unhybridized orbitals on the two carbons. A single bond is formed between the nitrogen and the middle carbon that consists of a s-type overlap of sp3 hybridized orbitals on the carbon and nitrogen.
- isomer III: A double bond is formed between the nitrogen atom in the middle and a carbon atom that consists of a s-type overlap of two sp2 hybridized orbitals on the two atoms and a p-type overlap of two unhybridized orbitals on the two. The carbon-nitrogen single bond consists of a s-type overlap of a sp3 hybridized orbital on the carbon atom with a sp2 hybridized orbital on the nitrogen atom.
d) What chemistry (i.e. types of reactions) would be possible for your isomer as a consequence of its electronic structure?
- isomer I: The lone pair on the nitrogen indicates that the isomer will function as a weak base. The double bond indicates that the isomer will readily react with electron-greedy reagents called electrophiles.
- isomers III: The lone pair on the nitrogen indicates that the isomer will function as a weak base. The double bond indicates that the isomer will readily react with electron-greedy reagents called electrophiles.
Q11
Answer the following questions for the set of molecules: \(H_2^+\), \(H_2\), \(H_2^-\), \(H_2^{-2}\).
- Write down the electronic configuration of each molecule.
- Arrange the molecules in order of increasing bond length and bond dissociation energy
- For each molecule, sketch a graph of the electronic energy versus the H-H interatomic distance. The 4 graphs should been scaled identically so that they can be compared with one another.
- Which of the molecules will be paramagnetic? Also answer questions a-d for the set \(He_2^{2+}\), \(He_2^+\), and \(He_2\).

