5.11: Spin-Spin, J-Coupling or indirect dipole-dipole coupling (all the same phenomenon)
- Page ID
- 369993
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)By a mechanism not discussed here, the magnetic moment induced by nearby nuclear spin can be felt as a contribution to the magnetic field at a nuclear undergoing resonance. Thus if the B spin had two orientations (I=1/2 had only two possible orientations), two possible local fields at A would results and this resonance would be split into two resonances - \(J\).
The frequency, \(J_{AB}\) is independent of \(H_o\) (internal forces only), while δ for two different nuclear is proportional to \(H_0\). That is how we can tell chemical shifts splitting from spin-spin splitting.
The resonance frequencies (or field position) of a proton is influenced not only by the shielding parameter, but also by the orientations of other nuclear spins in the molecules with which it is communication (like a correlation length for UV excitations). The interaction between nuclear spins in a molecule is called spin-spin coupling. This leads to structure in the NMR spectra in addition to the intrinsic chemical shift differences discussed above.
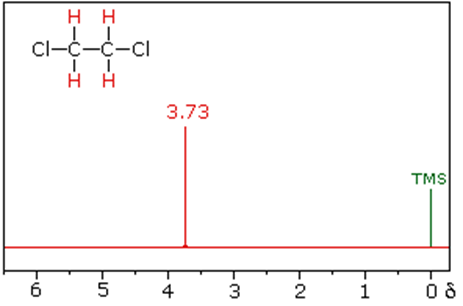
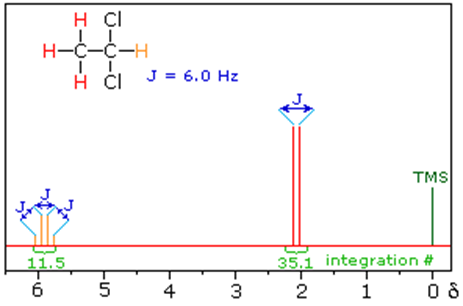
1,2-dichloroethane 1,1-dichloroethane
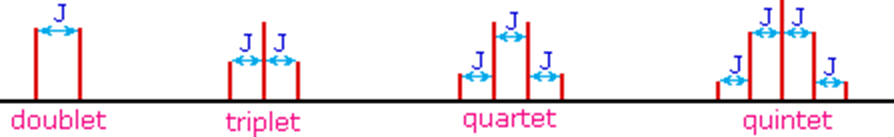
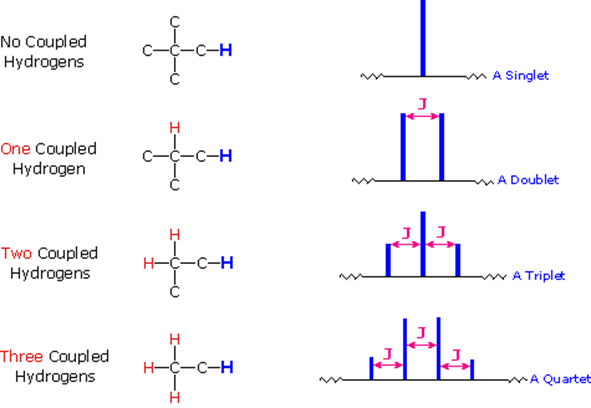
The splitting patterns found in various spectra are easily recognized, provided the chemical shifts of the different sets of hydrogen that generate the signals differ by two or more ppm. The patterns are symmetrically distributed on both sides of the proton chemical shift, and the central lines are always stronger than the outer lines. The most commonly observed patterns have been given descriptive names, such as doublet (two equal intensity signals), triplet (three signals with an intensity ratio of 1:2:1) and quartet (a set of four signals with intensities of 1:3:3:1). Four such patterns are displayed in the following illustration. The line separation is always constant within a given multiplet, and is called the coupling constant (J). The magnitude of J, usually given in units of Hz, is magnetic field independent.
The fine structure pattern of CH3 protons is due to the fact that the CH3 protons sense different orientations of the nearby CH2 protons (through bond coupling) in the field.
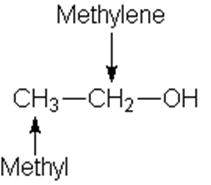
The coupling constant in Hz is JH-C-C-H or 3JH-H (superscript indicates that the coupled atoms are 3 bonds apart). The CH2 protons are split by the same coupling constant 3JH-H into a quartet with an intensity ratio of 1:3:3:1. There are two methylene protons of them, and each can have one of two possible orientations (aligned with or opposed against the applied field). This gives a total of four possible states;

In the first possible combination (left), spins are paired and opposed to the field. This has the effect of reducing the field experienced by the methyl protons; therefore a slightly higher field is needed to bring them to resonance, resulting in an upfield shift. Neither combination of spins opposed to each other has an effect on the methyl peak. The spins paired in the direction of the field produce a downfield shift. Hence, the methyl peak is split into three, with the ratio of areas 1:2:1.
Similarly, the effect of the methyl protons on the methylene protons is such that there are eight possible spin combinations for the three methyl protons;

Out of these eight groups, there are two groups of three magnetically equivalent combinations. The methylene peak is split into a quartet. The areas of the peaks in the quartet have the ration 1:3:3:1.
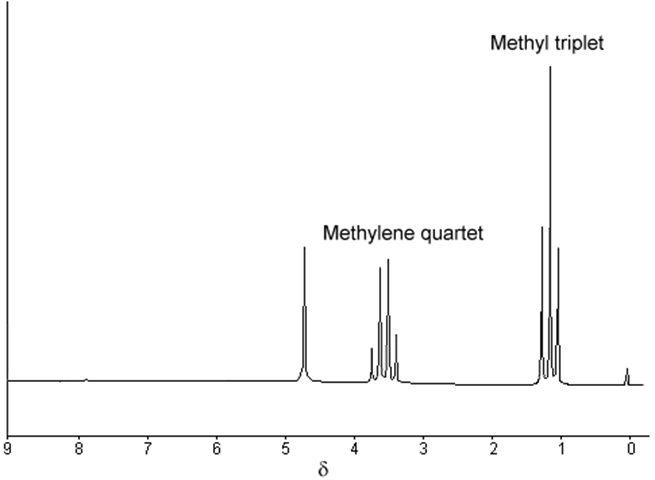
The spin mulitplet pattern of NMR spectra contain much structure info, as witnessed by C2H5OH. The intensity patterns of groups of equivalent nuclei of I=1/2 that split a resonance can be obtains by Pascal’s triangle (handy way to remember binomial coefficients):
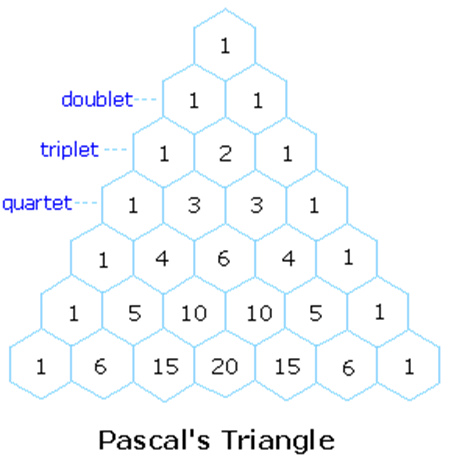
Any number is given as the sum of the two adjacent numbers on the preceding row. Each row is the number of nuclei equivalent protons are coupled to starting at 0.
Non I=1/2 splitting
The intensities of the individual lines is given by the spin I of the coupling nucleus (not the observed one). The relative intensities of the lines can be derived from Pascal's triangle and similar arrays:

When done properly, there are 12 distinct main transitions in the CH2 group and 13 in the CH3 group of ethanol, but all of them are never resolved. The distance between the different transitions depends on the magnetic field in such a way that at higher fields they are too close to be observed and several transitions often contribute to the same peak.
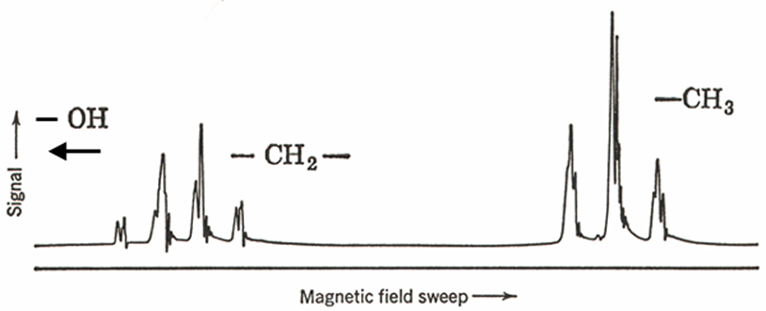
Higher resolution showing greater splitting in the bands in ETOH
Measureable J’s are usually observed between protons separated by up to 3-bands. 4 bands and > coupling are usually not observed. So splitting of CH3 by OH (four bands) is not seen (with the exception of aromatic molecules).
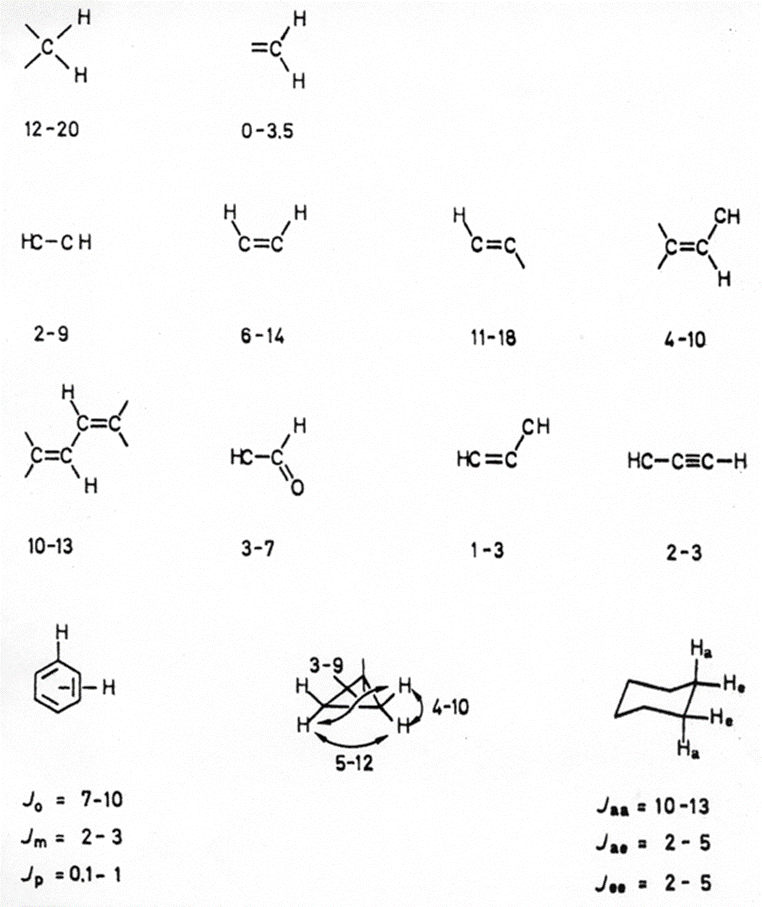
Coupling Constants (I): 2J(H,H) and 3J(H,H) Coupling Constants of Organic Compounds
The following is a list of H,H coupling constants observed for different structural fragments. Many different fragments give coupling constants in the range of 2-10 Hz so that coupling constants in this range are of limited value as a diagnostic tool for structure elucidation.
- Nuclei having the same chemical shift do not exhibit spin-splitting. They may actually be spin-coupled, but the splitting cannot be observed directly.
- Nuclei separated by three or fewer bonds (e.g. vicinal and geminal nuclei ) will usually be spin-coupled and will show mutual spin-splitting of the resonance signals (same J's), provided they have different chemical shifts. Longer-range coupling may be observed in molecules having rigid configurations of atoms.
- The magnitude of the observed spin-splitting depends on many factors and is given by the coupling constant J (units of Hz). J is the same for both partners in a spin-splitting interaction and is independent of the external magnetic field strength.
- The splitting pattern of a given nucleus (or set of equivalent nuclei) can be predicted by the n+1 rule, where n is the number of neighboring spin-coupled nuclei with the same (or very similar) Js. If there are 2 neighboring, spin-coupled, nuclei the observed signal is a triplet ( 2+1=3 ); if there are three spin-coupled neighbors the signal is a quartet ( 3+1=4 ). In all cases the central line(s) of the splitting pattern are stronger than those on the periphery. The intensity ratio of these lines is given by the numbers in Pascal's triangle. Thus a doublet has 1:1 or equal intensities, a triplet has an intensity ratio of 1:2:1, a quartet 1:3:3:1 etc.
The interpretation of the 1st order spectra is relatively straightforward. Not so for spectra in which the relative chemical shifts are similar to JAB. These give very complex spectra that are hard to interpret. The best solution is to use the strongest applied field as possible to induce the greatest chemical shifts allowing for separation of δ from J (bigger is better).
Spin-spin coupling isn’t limited to protons with protons, but will all nuclei that have magnetic fields (for example 13C will couple with 1H or 13C with 14N).
Distance Dependence of Coupling Constants
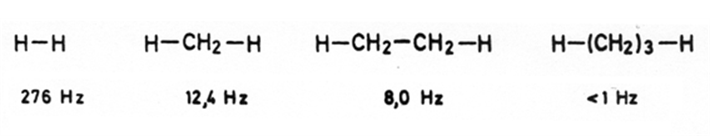
While 1J, 2J or 3J coupling is commonly observed for H,H coupling, coupling constants 4J or higher are usually too small to be observed.
A notable exception are 4J coupling constants in rigid frameworks:
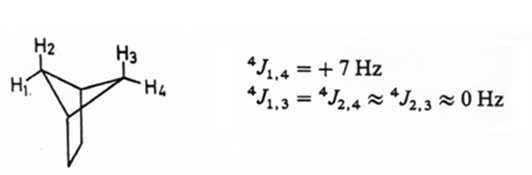
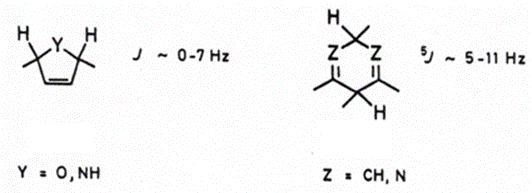
Geometric considerations in J Coupling
The 3J(H,H) coupling constants of aliphatic H-C-C-H fragments depend on the dihedral angle. The quantitative relationship is given by the Karplus equation, named after Martin Karplus (Harvard), which describes the correlation between 3J-coupling constants and dihedral torsion angles in nuclear magnetic resonance spectroscopy:
\[J(\phi)=A \cos ^{2} \phi+B \cos \phi+C \nonumber \]
where J is the 3J coupling constant, φ is the dihedral angle, and A, B, and C are empirically-derived parameters whose values depend on the atoms and substituents involved. The relationship may be expressed in a variety of equivalent ways e.g., involving cos2 φ rather than cos 2φ —these lead to different numerical values of A, B, and C but do not change the nature of the relationship.
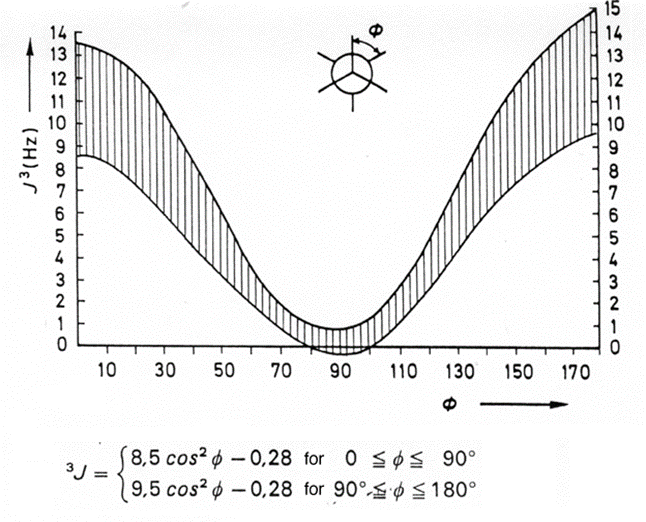
The Karplus equation is extremely important tool for the elucidation of 3D structures via NMR methods.
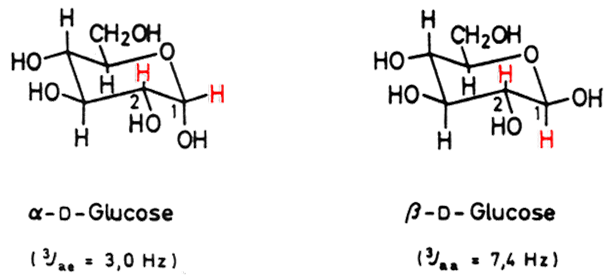
The Karplus equation is a useful tool to distinguish between the equatorial and axial protons present in many natural products. In the following example, the 3J(H,H) coupling constants used to distinguish between a-D-Glucose and b-D-Glucose: