5.12: 13-C NMR Spectroscopy
- Page ID
- 370462
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The carbon-12 nucleus does not have a nuclear spin, but the carbon-13 (13C) nucleus does due to the presence of an unpaired neutron. Carbon-13 nuclei make up approximately one percent of the carbon nuclei on earth. Therefore, carbon-13 NMR spectroscopy will be less sensitive (have a poorer SNR) than hydrogen NMR spectroscopy. Therefore, only the few 13C nuclei present resonate in the magnetic field, although this can be overcome by isotopic enrichment of e.g. protein samples. In addition, the gyromagnetic ratio (6.728284 107 rad T−1 s−1) is only 1/4 that of 1H, further reducing the sensitivity. The overall signals of 13C is about 4 orders of magnitude lower than 1H.
Chemical Shifts
As with proton NMR, the chemical shift reference standard for 13C is the carbons in tetramethylsilane (TMS), whose chemical shift is set to 0.0 ppm.
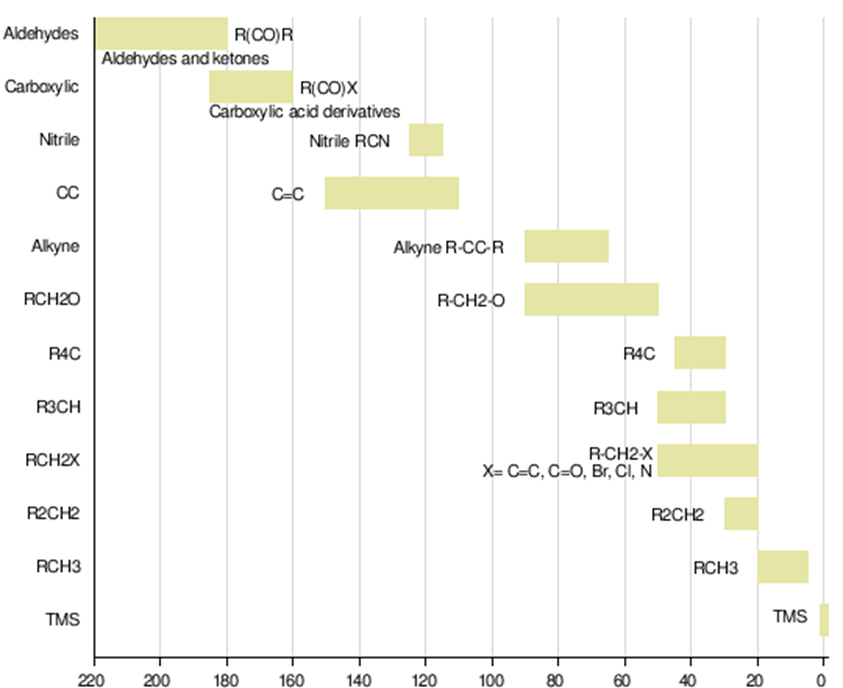
Chemical shift depends on the net magnetic field felt by the nuclei (H,C). The net magnetic field, in turn, depends upon the electron density possessed by the particular atom (Shielding constant). In the case of hydrogen, there is just a single electron and by coordination with others (H-F, CH3-F etc) leads to the slight change in electron density around the hydrogen nuclei, therefore, results in the small range of chemical shift. However, carbon having six electrons, being tetravalent, as well as attached to diverse functionalities leads to the considerable change in electron density around the carbon nuclei thereby, possess broader range of chemical shift values.
This is a simple example of a C-13 NMR spectrum. do not worry about the scale for now - we'll look at that in a minute.
![c13ethanol[1].gif](https://chem.libretexts.org/@api/deki/files/123570/c13ethanol%255B1%255D.gif?revision=1)
The NMR spectra on this page have been produced from graphs taken from the Spectral Data Base System for Organic Compounds (SDBS) at the National Institute of Materials and Chemical Research in Japan. It is possible that small errors may have been introduced during the process of converting them for use on this site, but these won't affect the argument in any way.
There are two peaks because there are two different environments for the carbons. The carbon in the CH3 group is attached to 3 hydrogens and a carbon. The carbon in the CH2 group is attached to 2 hydrogens, a carbon and an oxygen. The two lines are in different places in the NMR spectrum because they need different external magnetic fields to bring them in to resonance at a particular radio frequency.
This is the C-13 NMR spectrum for 1-methylethyl propanoate (also known as isopropyl propanoate or isopropyl propionate).
![c13isopropprop[1].gif](https://chem.libretexts.org/@api/deki/files/123571/c13isopropprop%255B1%255D.gif?revision=1)
This time there are 5 lines in the spectrum. That means that there must be 5 different environments for the carbon atoms in the compound. Is that reasonable from the structure?
![isopropprop[1].gif](https://chem.libretexts.org/@api/deki/files/123572/isopropprop%255B1%255D.gif?revision=1)
Well - if you count the carbon atoms, there are 6 of them. So why only 5 lines? In this case, two of the carbons are in exactly the same environment. They are attached to exactly the same things. Look at the two CH3 groups on the right-hand side of the molecule.
You might reasonably ask why the carbon in the CH3 on the left is not also in the same environment. Just like the ones on the right, the carbon is attached to 3 hydrogens and another carbon. But the similarity is not exact - you have to chase the similarity along the rest of the molecule as well to be sure.
The carbon in the left-hand CH3 group is attached to a carbon atom which in turn is attached to a carbon with two oxygens on it - and so on down the molecule. That's not exactly the same environment as the carbons in the right-hand CH3 groups. They are attached to a carbon which is attached to a single oxygen - and so on down the molecule.
Heteronuclear Coupling
Many organic molecules contain 1H as only NMR active nucleus and observed couplings are accordingly those between neighboring protons, the situation is more complex if other high abundance I = 1/2 nuclei are present (e.g., 13C, 19F and 31P). The below spectrum is a 1H broadband decoupled 13C spectrum (shorthand notation: 13C {1H }) of trifluoroacetic acid that shows "hydrogen like " coupling (two quartets).
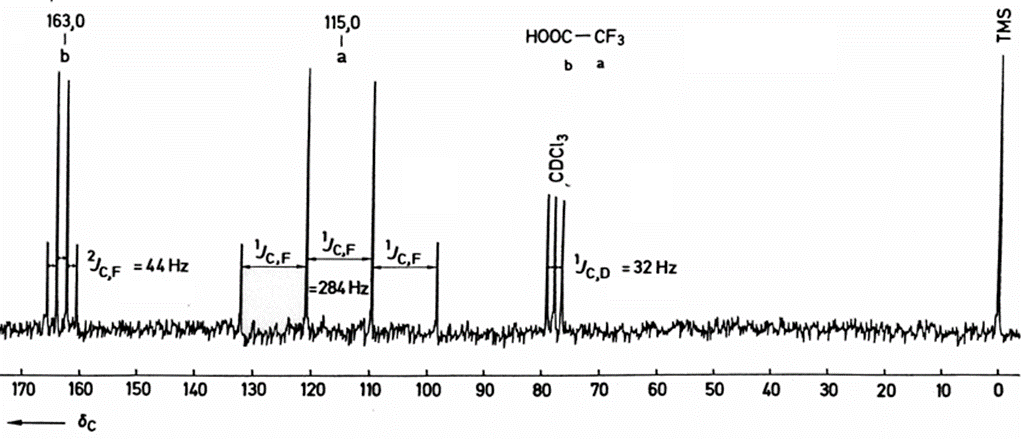
Quadrupolar nuclei (e.g., 2H) can also couple to I=1/2 nuclei, but is usually detected by measuring the quadrupolar nucleus, but rarely by measuring the I=1/2 nucleus (lifetime, T2, issue). J =1/2 to J =1/2 is generally observed unless fast exchange disrupts the bond (loss of through-bond coupling)
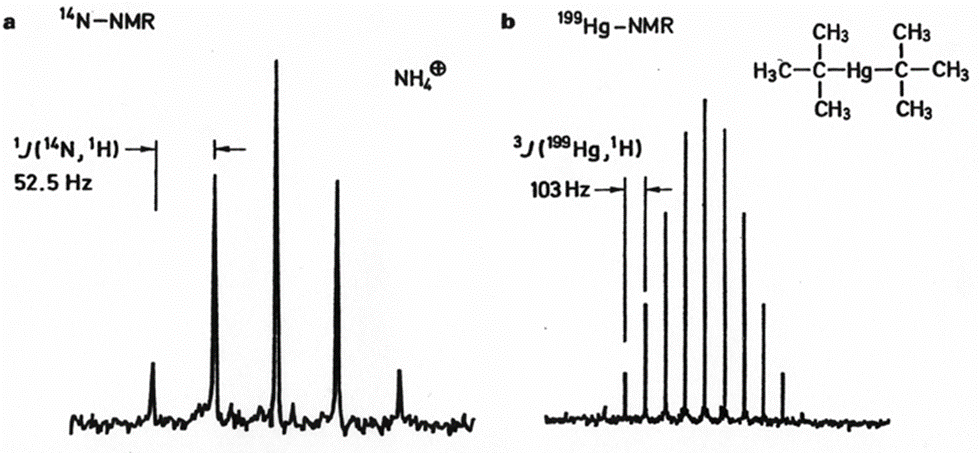
14N NMR Spectrum of ammonium ions (a) and 199Hg NMR spectrum of di-tert-butylmercury (b); Spectrometer frequency 4.33 and 14.3 MHz. b: only 11 of the 19 expected lines are actually observed.
J-coupling constants between carbon and hydrogen are typically from 100 to 250 Hz. To suppress these couplings, which would otherwise complicate the spectra and further reduce sensitivity, carbon-13 NMR spectra are often "proton decoupled" to remove the signal splitting. Typically, 13C-13C couplings can be ignored due to the low natural abundance of 13C (unless specifically synthesized that way in the lab). Hence in contrast to typical proton NMR spectra which show multiplets for each proton position, carbon NMR spectra typically show a single peak for each chemically non-equivalent carbon atom.

