1: Safety Lab
- Page ID
- 338889
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)General Information
Chemical Safety is one of the most important topics covered in the general chemistry laboratory. There are multiple goals in the safety curriculum. First are the obvious goals of keeping students safe during the lab and not creating hazards for others. The second is to develop the proper protocols and procedures for performing experimental activities so that when the student enters advanced courses or the work force, they have the skills needed to function in a safe and prudent manner. But there is another goal for safety, and this deals with safety literacy in the digital age, that is, in this age of instant information, where people can post false claims online, it is important that students develop the skills to evaluate chemical safety information. Thus we will include activities in evaluating online information and the importance of understanding data provenance before assessing the validity of online information. That is, where did you get this data? Is it a valid source, and did it provide a trail for you to further understand where the data came from. We will start this module by looking at the Chemical Hygiene Plan at UALR.
Chemical Hygiene Plan
Every university has a CHP (Chemical Hygiene Plan) as required by OSHA standard 29 CFR 1910.1450 and a CHO (Chemical Hygiene Officer) who is responsible for its implementation, and UALR's CHP can be found at the Facilities Management Web Page. Within the CHP are a set of standard SOPs (Safe Operating Procedures) that represent the minimum safe practices for the handling of hazardous chemicals and the operation of equipment (like how to secure a compressed gas tank). Every research lab at the university is required to develop and maintain SOPs for the laboratory practices that are performed within their labs, and here is a link to the template for developing laboratory specific SOPs. The CHP also defines the PPE (Personal Protective Equipment) needed to perform work in a laboratory, and in the university teaching laboratory the instructor would be responsible for ensuring students abide by the established SOPs, and wear proper PPE, like safety glasses and closed toe shoes (no flip-flops in the chemistry laboratory), and do not perform any unauthorized experiments.
Chemical Safety Resources
Prudent Practices
The National Research Council of the National Academies of Sciences has published a book "Prudent Practices in the Laboratory" that can be downloaded for free and has a wealth of information on chemical safety, including a copy of OSHA's Laboratory Standard (29 CFR 1910.1450). There is also an accompanying zip file of a CD that contains Laboratory Chemical Safety Summaries (LCSS) and additional information. LCSS contain chemical and toxicological information from a variety of sources.

Chemical Safety Board
The Chemical Safety Board (CSB) of the U.S. government became operational in 1998 and is involved with investigating and identifying the cause or causes of industrial accidents. The members of the board are president and confirmed by the U.S. Senate. It operates independently of the EPA and OSHA, which are involved with developing and enforcing environmental, health and safety laws.
 Figure \(\PageIndex{2}\): Logo of the U.S. Chemical Safety Board (https://www.csb.gov/).
Figure \(\PageIndex{2}\): Logo of the U.S. Chemical Safety Board (https://www.csb.gov/).
UN GHS
OSHA's laboratory standard is actually integrated into the United States implementation of the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (GHS) and the 8th edition can be downloaded as a PDF. Within the GHS are the requirements for Safety Data Sheets (SDS) which have superseded the MSDS (Material Safety Data Sheets) that are required for any chemical transported or sold within the US. SDS as actually developed by the manufacturers of chemicals and will have a legal disclaimer, and the regulations state what kind of material must be provided, but they do not validate that the information is correct. Likewise, the GHS labeling system does not define what labels should be on a chemical, that is up to the laws and regulations of a country the chemical is in, but it says the same label means the same thing in all countries. Before the UN GHS things were a real mess, in fact in the US you would use OSHA regulations when acquring and using chemicals, DOT (Department of Transportation) regulations when transporting and EPA (Environmental Protection Agency) when disposing, and they often had different symbols for the same hazard, as described in the 2002 article by Dawn Lee, From Cradle to Grave: How to Manage Chemicals in Between.


PubChem LCSS
The National Institute of Health's (NIH) National Library of Medicine's (NLM) PubChem have developed LCSS that model the LCSS of the NRC, but extract data from multiple chemical compound databases. This is a very valuable resource for finding safety information on chemicals. One of the greatest values of PubChem LCSS is that they maintain the data provenance for all the information, that is, you can find the source of the information. This means you can often find contradicting information as different sources may have posted different information. PubChem LCSS makes it very easy to survey information on a specific chemical from multiple resources and having this skill is very important.
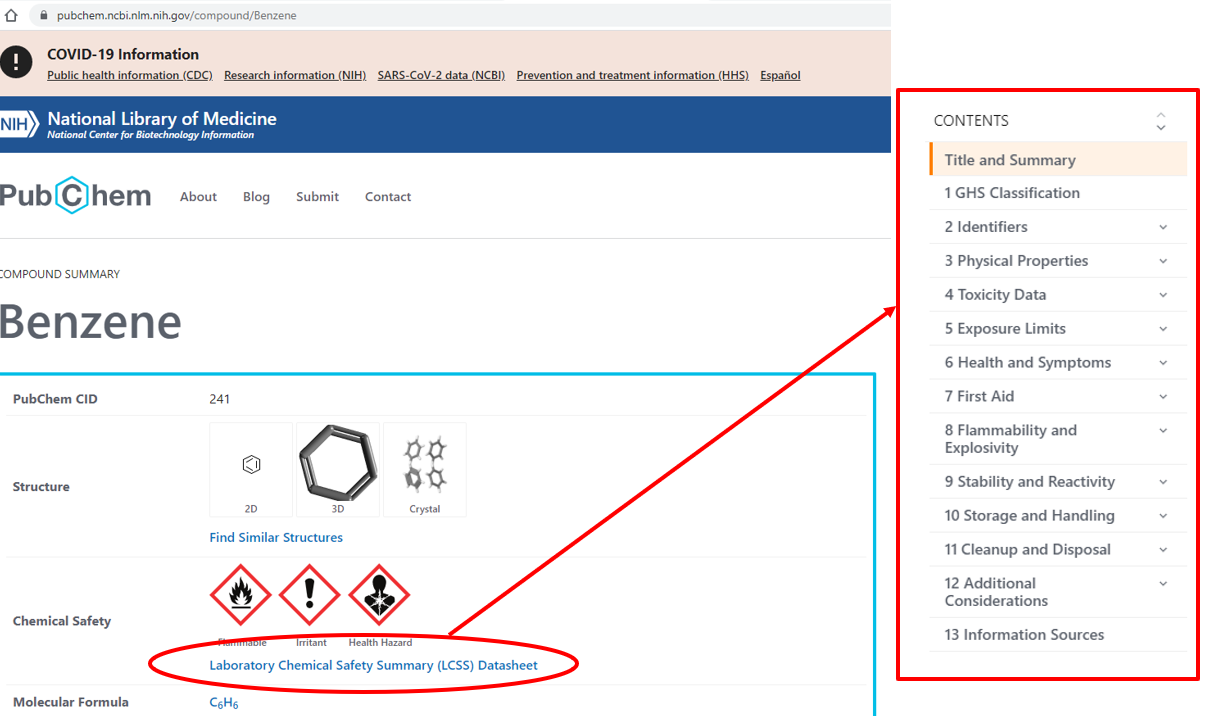
Figure \(\PageIndex{1}\): When you search a chemical in PubChem you are taken to the "Compound Summary Page," which has a link to that chemical's LCSS (left part of image). If you click LCSS you are taken to content related to chemical health and safety, the red box is the LCSS table of contents for Benzene. (Bob Belford; CC0)
Throughout the semester you will be looking up information related to chemical safety in both LCSS and manufacturer's SDS.
Safety in the Laboratory
You are required to watch this video and answer the questions of the Safety Video Quiz.
The following images show you the actual lab you will be working in. When you start working in a lab make sure you know where all the safety equipment is located and how to use it.

Let's take a closer look at the eyewash. To operate the eye wash open the dust covers and push the handle located immediately to the right from it. Just in case some debris has settled on the water spout you should always flush out the water from the nozzle before flushing your eye with it.

If you spill a chemical like HCl on your cloths you need to quickly take them off and rinse yourself with the safety shower. We typically work with dilute solutions and the experiments are designed to minimize risk, but in the event you are exposed to a caustic chemical you must rinse with copious amounts of water.

The lab blanket is for fires. Never wrap it vertically while you are standing as it can have a chimney effect and the flame can rise to your head. You "stop, drop and roll", that is you get down horizontally and wrap the fire blanket around you to smother the flames.

Always remember to dispose of chemicals in the way indicated in your lab manual. In the lab you will find waste jars labeled for each experiment. Contact your instructor if a jar is full.

Always remember to ask your instructor if you're not sure about something!
Laboratory Etiquette and Safety Rules
During the beginning of each experiment we will go over procedures and safety hazards. If you miss this portion of a lab, you will not be allowed to perform any wet work associated with the lab. You will also not be allowed to use your partner's data. It is imperative that you come to the lab on time.
Safety Agreement
During the first lab you will be given the following form and sign it, agreeing to abide by these protocols.
- Read the entire experiment before coming to the laboratory session. The pre-laboratory activities must be done before lab and submitted through the ADAPT homework system within LibreText. are designed to familiarize you with the material necessary to understand the experiment. These questions are to be answered before you come to the first lab session for a given experiment and handed to your lab instructor before the pre-lab lecture.
- You may only work in the laboratory during scheduled periods. Students must be supervised at all times. Do not unlock your lab drawer until your lab instructor is present.
- No horseplay or practical jokes in the lab. Unauthorized experiments or deviations from the lab procedure are forbidden.
- Keep your desk and work space neat and clean. Solids are disposed of in the waste basket at the end of your bench, NOT IN THE SINK. Pour liquids into the sink followed by running water. At the end of each lab period, clean, dry and put away all equipment. Wash and dry the lab bench top.
- Record your lab data and observations directly into your lab manual. Do not use scratch paper or other pages.
- You are expected to be on time to pre-lab and lab and stay until the end of the lab session. If you leave lab early for any reason, be sure that your lab instructor initials your data sheet.
- Any accidents, injuries, corrosive spills or other irregularities must be immediately reported to your instructor. Observe all safety rules.
- Books, backpacks, coats, and purses must be stored in the shelves during the lab. Only your lab manual, textbook and calculator are to taken to your workspace.
- The chemicals and special equipment for each experiment will be available on a cart in your lab. DO NOT TAKE THE CHEMICAL STOCK BOTTLES TO YOUR WORK SPACE. Bring your clean, dry containers to the cart to obtain just enough of the chemical for your experiment. Replace all lids on stock bottles, even if the person behind you wants the same chemical.
- . If you take more chemical than you need, DO NOT RETURN IT TO OUR STOCK BOTTLES; IT WOULD BE CONTAMINATED. Share any excess with another student.
- Read all labels twice. Once to determine the name, again to confirm the concentration. Serious accidents can happen if the wrong chemical or wrong concentration is used.
- Be very careful not to mix lids or stoppers; this can lead to contamination.
- Never taste a chemical in lab. If instructed to describe an odor, be sure to gently fan the vapors toward your nose.
- When heating in a test tube, do not point it toward yourself or another student.
- Always pour acid into water slowly while stirring. NEVER pour water into acid.
- If an experiment evolves noxious or poisonous gases, be sure to conduct them in the fume hood.
- Never use acetone, ether or other flammable chemicals near open flames.
- Never leave spilled chemicals or water anywhere in the lab. Paper towels and mops are available to clean up your spills. WE DO NOT HAVE MAID SERVICE.
- Never pipet liquids by mouth. Use a pipet bulb.
- Do not bring any food or drink into the lab. No eating or drinking in the lab for your protection.
- Do not sit on the lab benches.
- Safety glasses must be worn on your eyes the entire time you are in the lab. You must keep them on if you remain in lab after you have finished your experiment.
- Wear comfortable clothes and shoes. Tie loose hair back.
- Use a fire extinguisher to put out paper fires. If your clothing catches on fire, use the fire blanket. Use the shower if you spill corrosive material on large parts of your body. Use the eye wash if any chemical gets in your eyes.
- Locate the following safety equipment in your lab and memorize their locations: fire extinguisher, fire blanket, eye wash, shower, fire alarm, and fume hood.
- Acid spills are neutralized with sodium bicarbonate. Base spills are neutralized with dilute acetic acid.
- Be sure to take advantage of the full precision of your laboratory devices.


