12: Solids
- Page ID
- 205366
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Prelude
Crystal Lattices and Unit Cells
Exercise \(\PageIndex{2a}\)
Crystalline solids differ from amorphous solids by _____.
- Substantial intermolecular attractive forces
- A long-range repeating pattern of atoms, molecules, or ions
- Atoms, molecule, or ions that are close together
- Much larger atoms, molecules, or ions
- No orderly structure
- Answer
-
b. A long-range repeating pattern of atoms, molecules, or ions
Exercise \(\PageIndex{2b}\)
______ is a unit cell with all sides the same length and all angles equal to 90° that has lattice points only at the corners.
- Body-centered cubic
- Face-centered cubic
- Monoclinic
- Primitive cubic
- Spherical cubic
- Answer
-
d. Primitive cubic
Exercise \(\PageIndex{2c}\)
What is the fraction that each corner atom takes up in a face-centered cubic unit cell?
- 1
- 1/2
- 1/4
- 1/8
- 1/16
- Answer
-
d. 1/8
Exercise \(\PageIndex{2d}\)
A face-centered cubic unit cell contains how many atoms?
- Answer
-
Four
Exercise \(\PageIndex{2e}\)
Based on sodium chloride structure, which of the following cannot form a solid lattice?
- NaBr
- LiF
- RbI
- CuO
- CuCl2
- Answer
-
e. CuCl2
Exercise \(\PageIndex{2f}\)
What type of solid is held together by dispersion, dipole-dipole or hydrogen bonds?
- Ionic
- metallic
- molecular
- covalent network
- Answer
-
c. molecular
Exercise \(\PageIndex{2g}\)
What type of compounds are held together by covalent bonds? (there can be more than one correct answer)
- ionic
- metallic
- molecular
- covalent network
- Answer
-
d. covalent network and molecular
Exercise \(\PageIndex{2h}\)
Solid Iodine is a ____type of substance:
- ionic lattice
- metallic
- molecular
- covalent network
- Answer
-
c. molecular
Exercise \(\PageIndex{2i}\)
Diamond lattices are a ___ type of substance
- ionic crystal
- metallic
- molecular
- network covalent
- Answer
-
d. network covalent
Exercise \(\PageIndex{2j}\)
How many basic crystal systems are there?
- 3
- 4
- 6
- 7
- Answer
-
d. 7
Exercise \(\PageIndex{2k}\)
Which is not a type of cubic unit cell?
- tetragonal
- body-centered
- face centered
- primitive
- Answer
-
a. tetragonal
Exercise \(\PageIndex{2l}\)
Which type of cubic unit cell is the least efficient in packing?
- primitive
- body-centered
- face-centered
- none of the above
- Answer
-
a. primitive
Exercise \(\PageIndex{2m}\)
Which type of cubic unit cell is most efficient in packing?
- primitive
- body-centered
- face-centered
- none of the above
- Answer
-
c. face-centered
Exercise \(\PageIndex{2n}\)
Primitive, Face-Centered & Body-Centered Cubic Cells have respective coordination numbers of
- 1,2,4
- 2,4,6
- 6,8,12
- 6,12,8
- Answer
-
d. 6,12,8
Exercise \(\PageIndex{2o}\)
In a face-centered cubic cell, what portion of the volume of each atom or ion on the face of a unit is within the unit cell?
- Answer
-
1/2 of the atom is within the unit cell
Exercise \(\PageIndex{2p}\)
Gallium crystallizes in a primitive cubic unit cell. What is the radius of the Ga atom in Angstroms if the length of the unit cell edge is 3.70Å?
- Answer
-
\[l=2r\]
\[r=\frac{l}{2}=\frac{3.70\AA }{2}=1.85 \AA\]
Exercise \(\PageIndex{2q}\)
Potassium metal crystallizes in a body-centered cubic unit cell. What is the radius of the K atom in Angstroms if the length of the unit cell edge is 5.31 Å?
- Answer
-
\[b^{2}=a^{2}+a^{2}\]
\[b=\sqrt{2a^{2}}=\sqrt{2*(5.31)^{2}}=7.51\AA\]
\[c^{2}=a^{2}+b^{2}\
\[c=\sqrt{a^{2}+b^{2}}=\sqrt{(5.31)^{2}+(7.51)^{2}}=9.20\AA\]
\[c=4r\]
\[r=\frac{c}{4}=\frac{9.20\AA }{4}=2.30\AA\]
Exercise \(\PageIndex{2r}\)
What is the radius of a copper atom in Angstroms if the length of the unit cell edge is 5.34Å? Copper has a face-centered cubic structure.
- Answer
-
\[c^{2}=a^{2}+a^{2}\]
\[c=\sqrt{2a^{2}}=\sqrt{2*\left ( 5.34\AA \right )^{2}}=7.55 \AA \]
\[r=\frac{c}{4}=\frac{7.55\AA }{4}=1.89 \AA \]
Exercise \(\PageIndex{2s}\)
Silver has a density of 10.5g/cm3 and forms an FCC structure. What is the atomic radius of silver in Angstroms? Assume that nearest-neighbor atoms contact each other.
- Answer
-
- Calculate volume of unit cell
\[\left ( \frac{4\,atoms}{unit\,cell} \right )\left ( \frac{1\,mol}{6.022*10^{23}\,atoms} \right )\left ( \frac{107.87\,g}{1\,mol} \right )\left ( \frac{1\,cm^{3}}{10.5\,g} \right )=6.82*10^{-23}\,cm^{3}\]
- Calculate length of unit cell
\[V=l^{3}\]
\[l=\sqrt[3]{V}\]
\[l=\sqrt[3]{V}=\sqrt[3]{6.82*10^{-23}cm^{3}}=4.09*10^{-8}cm*\left ( \frac{10^{10}\AA }{100cm} \right )=4.09\AA\]
- Calculate radius of unit cell
\[c^{2}=a^{2}+a^{2}\]
\[c=\sqrt{2a^{2}}=\sqrt{2*\left ( 4.09\AA \right )^{2}}=5.78\AA\]
\[r=\frac{c}{4}=\frac{5.78\AA }{4}=1.44\AA\]
Exercise \(\PageIndex{2t}\)
An unknown element has a density of 11.07g/mL and forms a Simple Cubic Cell. What is the atomic radius of the unknown element in Angstroms? (unknown element has molar mass of 207.2g/mol)
- Answer
-
- Calculate volume of unit cell
\[\left ( \frac{1 atom}{unit cell} \right )\left ( \frac{1 mol}{6.022*10^{23}atoms} \right )\left ( \frac{207.2g}{1mol} \right )\left ( \frac{1mL}{11.07g} \right )=3.11*10^{-23}cm^{3}\]
- Calculate length of unit cell
\[V=l^{3}\]
\[l=\sqrt[3]{V}\]
\[l=\sqrt[3]{V}=\sqrt[3]{3.11*10^{-23}cm^{3}}=3.14*10^{-8}cm*\left ( \frac{10^{10}\AA }{100cm} \right )=3.14\AA\]
- Calculate radius of unit cell
\[l=2r\]
\[r=\frac{l}{2}=\frac{3.14\AA }{2}=1.57\AA\]
Exercise \(\PageIndex{2u}\)
Tungsten has a density of 19.25g/cm3 and forms a BCC structure. What is the atomic radius of tungsten in Angstroms?
- Answer
-
- Calculate volume of unit cell
\[\left ( \frac{2\,atoms}{unit\,cell} \right )\left ( \frac{mol}{6.022*10^{23}\,atoms} \right )\left ( \frac{183.94\,g}{mol} \right )\left ( \frac{1\,cm^{3}}{19.25\,g} \right )=3.17*10^{-23}\,cm^{3}\]
-
Calculate length of unit cell
\[V=l^{3}\]
\[l=\sqrt[3]{V}\]
\[l=\sqrt[3]{V}=\sqrt[3]{3.17*10^{-23}cm^{3}}=3.17*10^{-8}cm*\left ( \frac{10^{10}\AA }{100cm} \right )=3.17\AA=a\]
- Calculate radius of unit cell
\[b^{2}=a^{2}+a^{2}\]
\[b=\sqrt{2a^{2}}=\sqrt{2*(3.17)^{2}}=4.48\AA\]
\[c^{2}=a^{2}+b^{2}\]
\[c=\sqrt{a^{2}+b^{2}}=\sqrt{(3.17)^{2}+(4.48)^{2}}=5.49\AA\]
\[c=4r\]
\[r=\frac{c}{4}=\frac{5.49\AA }{4}=1.37\AA\]
Ionic Solids
Bonding in Metals and Semiconductors
Network and Amorphous Solids
Phase Diagrams
Exercise \(\PageIndex{6a}\)
A substance under normal conditions would rather sublime than melt if _____.
- Its critical point occurs at a pressure above atmospheric pressure
- Its critical point occurs at a temperature above room temperature
- Its critical temperature is above its normal boiling point
- Its triple point occurs at a pressure above atmospheric pressure
- Its triple point occurs at a pressure below atmospheric pressure
- Answer
-
d. Its triple point occurs at a pressure above atmospheric pressure
Exercise \(\PageIndex{6b}\)
If a phase diagram has a solid-liquid phase boundary line that has a negative slope (leans to left) the substance,
- Can go from solid to liquid, within a small temperature range, via the application of pressure
- Cannot be liquefied above its triple point
- Cannot go from solid to liquid by application of pressure at any temperature
- Melts rather than sublimes under ordinary conditions
- Sublimes rather than melts under ordinary conditions
- Answer
-
a. Can go from solid to liquid, within a small temperature range, via the application of pressure
Exercise \(\PageIndex{6c}\)
The critical temperature, on a phase diagram, is _____.
- The temperature above which a gas cannot be liquefied
- The temperature at which all these states are in equilibrium
- The temperature below which a gas cannot be liquefied
- The temperature required to cause sublimation of a solid
- The temperature required to melt a solid
- Answer
-
a. The temperature above which a gas cannot be liquefied
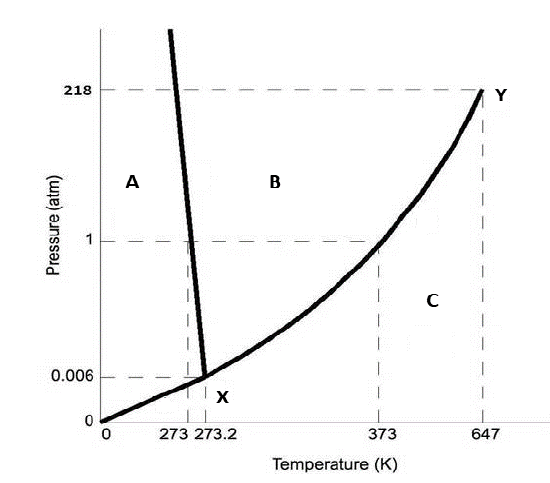
Figure 12.7.1: Use this figure to answer the following questions.
Exercise \(\PageIndex{6d}\)
The point X represents
- the critical point, where a solid, liquid and vapor can coexist
- The critical point where the two fluid phases cannot be distinguished
- The triple point, where a solid, liquid and vapor can coexist
- The triple point, where the fluid phases cannot be separated
- Answer
-
c. The triple point, where a solid, liquid and vapor can coexist
Exercise \(\PageIndex{6e}\)
The point Y in the figure represents
- the critical point, where a solid, liquid and vapor can coexist
- The critical point where the two fluid phases cannot be distinguished
- The triple point, where a solid, liquid and vapor can coexist
- The triple point, where the fluid phases cannot be separated
- Answer
-
b. The critical point where the two fluid phases cannot be distinguished
Exercise \(\PageIndex{6f}\)
Region A of the figure represents
- solid
- liquid
- vapor
- none of the above
- Answer
-
a. solid
Exercise \(\PageIndex{6g}\)
Region B of the figure represents
- solid
- liquid
- vapor
- none of the above
- Answer
-
b. liquid
Exercise \(\PageIndex{6.8}\)
Region C of the figure represents
- solid
- liquid
- vapor
- none of the above
- Answer
-
c. vapor
Exercise \(\PageIndex{6h}\)
The negative slope between regions A and B of figure 12.7.1 indicates:
- the solid is denser than the liquid
- the liquid is denser than the solid
- the vapor is denser than the liquid
- the vapor is denser than the solid
- Answer
-
b. the liquid is denser than the solid
Exercise \(\PageIndex{6i}\)
Figure 12.7.1 is consistent with a phase diagram for which compound
- carbon dioxide
- sodium
- water
- carbon dioxide and water
- Answer
-
c. water
Exercise \(\PageIndex{6j}\)
The compound in figure 12.7.1 sublimes at pressures:
- greater than deg O°C
- Pressures greater than 1.0 atm
- pressures between 0.0060 and 1.00 atm
- pressures less than 0.0060 atm
- Answer
-
d. pressures less than 0.0060 atm
Exercise \(\PageIndex{6k}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Moving left to right in region A represents
- freezing
- melting
- heating supercooled ice
- none of the above
- Answer
-
c. heating supercooled ice
Exercise \(\PageIndex{6l}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Adding heat to a substance in region A causes it to warm, what happens when you reach the line between region A & B?
- it boils
- it melts
- it freezes
- it continues to warm up
- Answer
-
b. it melts
Exercise \(\PageIndex{6m}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Moving left to right in region B represents
- melting
- boiling
- heating liquid water
- cooling liquid water
- Answer
-
c. heating liquid water
Exercise \(\PageIndex{6n}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Adding heat to a substance in region B causes it to warm, what happens when you reach the line between region B & C?
- it continues to warm
- it condenses
- it boils
- all of the above
- Answer
-
c. it boils
Exercise \(\PageIndex{6o}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Moving left to right in region C represents
- cooling water
- heating liquid water
- heating ice
- heating steam
- Answer
-
d. heating steam
Exercise \(\PageIndex{6p}\)
At what pressure can liquid, solid and gaseous water coexist?
- 218 atm
- 1.00 atm
- 0.0060 atm
- none of the above
- Answer
-
c. 0.0060 atm
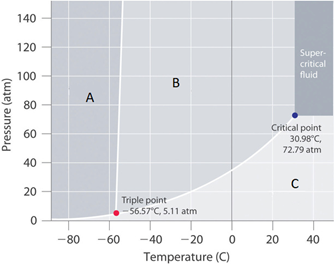
Exercise \(\PageIndex{6q}\)
Consider a -50°C isotherm for the compound in Figure 12.7.2. Moving from region A to C represents
- Condensation then Freezing
- Freezing then Condensation
- Melting then Vaporizing
- Vaporizing then Melting
- Answer
-
c. Melting then Vaporizing
Exercise \(\PageIndex{6r}\)
Consider a 5 atm isobar for the compound in Figure 12.7.2. Moving from region C to A represents
- Condensation
- Deposition
- Sublimation
- Vaporization
- Answer
-
b. Deposition
Exercise \(\PageIndex{6s}\)
What phase would this compound be in if the pressure and temperature were at room conditions?
- Answer
-
This substance would be a gas
Exercise \(\PageIndex{6t}\)
The positive slope between regions A and B of figure 12.7.2 indicates:
- the solid is denser than the liquid
- the liquid is denser than the solid
- the vapor is denser than the liquid
- the vapor is denser than the solid
- Answer
-
a. the solid is denser than the liquid
Exercise \(\PageIndex{6u}\)
Figure 12.7.2 is consistent with a phase diagram for which compound
- Carbon dioxide
- Carbon dioxide and water
- Sodium
- Water
- Answer
-
a. Carbon dioxide

