12: Solids
- Page ID
- 205366
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Prelude
Crystal Lattices and Unit Cells
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2a}\)
Crystalline solids differ from amorphous solids by _____.
- Substantial intermolecular attractive forces
- A long-range repeating pattern of atoms, molecules, or ions
- Atoms, molecule, or ions that are close together
- Much larger atoms, molecules, or ions
- No orderly structure
- Answer
-
b. A long-range repeating pattern of atoms, molecules, or ions
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2b}\)
______ is a unit cell with all sides the same length and all angles equal to 90° that has lattice points only at the corners.
- Body-centered cubic
- Face-centered cubic
- Monoclinic
- Primitive cubic
- Spherical cubic
- Answer
-
d. Primitive cubic
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2c}\)
What is the fraction that each corner atom takes up in a face-centered cubic unit cell?
- 1
- 1/2
- 1/4
- 1/8
- 1/16
- Answer
-
d. 1/8
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2d}\)
A face-centered cubic unit cell contains how many atoms?
- Answer
-
Four
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2e}\)
Based on sodium chloride structure, which of the following cannot form a solid lattice?
- NaBr
- LiF
- RbI
- CuO
- CuCl2
- Answer
-
e. CuCl2
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2f}\)
What type of solid is held together by dispersion, dipole-dipole or hydrogen bonds?
- Ionic
- metallic
- molecular
- covalent network
- Answer
-
c. molecular
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2g}\)
What type of compounds are held together by covalent bonds? (there can be more than one correct answer)
- ionic
- metallic
- molecular
- covalent network
- Answer
-
d. covalent network and molecular
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2h}\)
Solid Iodine is a ____type of substance:
- ionic lattice
- metallic
- molecular
- covalent network
- Answer
-
c. molecular
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2i}\)
Diamond lattices are a ___ type of substance
- ionic crystal
- metallic
- molecular
- network covalent
- Answer
-
d. network covalent
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2j}\)
How many basic crystal systems are there?
- 3
- 4
- 6
- 7
- Answer
-
d. 7
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2k}\)
Which is not a type of cubic unit cell?
- tetragonal
- body-centered
- face centered
- primitive
- Answer
-
a. tetragonal
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2l}\)
Which type of cubic unit cell is the least efficient in packing?
- primitive
- body-centered
- face-centered
- none of the above
- Answer
-
a. primitive
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2m}\)
Which type of cubic unit cell is most efficient in packing?
- primitive
- body-centered
- face-centered
- none of the above
- Answer
-
c. face-centered
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2n}\)
Primitive, Face-Centered & Body-Centered Cubic Cells have respective coordination numbers of
- 1,2,4
- 2,4,6
- 6,8,12
- 6,12,8
- Answer
-
d. 6,12,8
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2o}\)
In a face-centered cubic cell, what portion of the volume of each atom or ion on the face of a unit is within the unit cell?
- Answer
-
1/2 of the atom is within the unit cell
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2p}\)
Gallium crystallizes in a primitive cubic unit cell. What is the radius of the Ga atom in Angstroms if the length of the unit cell edge is 3.70Å?
- Answer
-
\[l=2r\]
\[r=\frac{l}{2}=\frac{3.70\AA }{2}=1.85 \AA\]
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2q}\)
Potassium metal crystallizes in a body-centered cubic unit cell. What is the radius of the K atom in Angstroms if the length of the unit cell edge is 5.31 Å?
- Answer
-
\[b^{2}=a^{2}+a^{2}\]
\[b=\sqrt{2a^{2}}=\sqrt{2*(5.31)^{2}}=7.51\AA\]
\[c^{2}=a^{2}+b^{2}\
\[c=\sqrt{a^{2}+b^{2}}=\sqrt{(5.31)^{2}+(7.51)^{2}}=9.20\AA\]
\[c=4r\]
\[r=\frac{c}{4}=\frac{9.20\AA }{4}=2.30\AA\]
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2r}\)
What is the radius of a copper atom in Angstroms if the length of the unit cell edge is 5.34Å? Copper has a face-centered cubic structure.
- Answer
-
\[c^{2}=a^{2}+a^{2}\]
\[c=\sqrt{2a^{2}}=\sqrt{2*\left ( 5.34\AA \right )^{2}}=7.55 \AA \]
\[r=\frac{c}{4}=\frac{7.55\AA }{4}=1.89 \AA \]
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2s}\)
Silver has a density of 10.5g/cm3 and forms an FCC structure. What is the atomic radius of silver in Angstroms? Assume that nearest-neighbor atoms contact each other.
- Answer
-
- Calculate volume of unit cell
\[\left ( \frac{4\,atoms}{unit\,cell} \right )\left ( \frac{1\,mol}{6.022*10^{23}\,atoms} \right )\left ( \frac{107.87\,g}{1\,mol} \right )\left ( \frac{1\,cm^{3}}{10.5\,g} \right )=6.82*10^{-23}\,cm^{3}\]
- Calculate length of unit cell
\[V=l^{3}\]
\[l=\sqrt[3]{V}\]
\[l=\sqrt[3]{V}=\sqrt[3]{6.82*10^{-23}cm^{3}}=4.09*10^{-8}cm*\left ( \frac{10^{10}\AA }{100cm} \right )=4.09\AA\]
- Calculate radius of unit cell
\[c^{2}=a^{2}+a^{2}\]
\[c=\sqrt{2a^{2}}=\sqrt{2*\left ( 4.09\AA \right )^{2}}=5.78\AA\]
\[r=\frac{c}{4}=\frac{5.78\AA }{4}=1.44\AA\]
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2t}\)
An unknown element has a density of 11.07g/mL and forms a Simple Cubic Cell. What is the atomic radius of the unknown element in Angstroms? (unknown element has molar mass of 207.2g/mol)
- Answer
-
- Calculate volume of unit cell
\[\left ( \frac{1 atom}{unit cell} \right )\left ( \frac{1 mol}{6.022*10^{23}atoms} \right )\left ( \frac{207.2g}{1mol} \right )\left ( \frac{1mL}{11.07g} \right )=3.11*10^{-23}cm^{3}\]
- Calculate length of unit cell
\[V=l^{3}\]
\[l=\sqrt[3]{V}\]
\[l=\sqrt[3]{V}=\sqrt[3]{3.11*10^{-23}cm^{3}}=3.14*10^{-8}cm*\left ( \frac{10^{10}\AA }{100cm} \right )=3.14\AA\]
- Calculate radius of unit cell
\[l=2r\]
\[r=\frac{l}{2}=\frac{3.14\AA }{2}=1.57\AA\]
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{2u}\)
Tungsten has a density of 19.25g/cm3 and forms a BCC structure. What is the atomic radius of tungsten in Angstroms?
- Answer
-
- Calculate volume of unit cell
\[\left ( \frac{2\,atoms}{unit\,cell} \right )\left ( \frac{mol}{6.022*10^{23}\,atoms} \right )\left ( \frac{183.94\,g}{mol} \right )\left ( \frac{1\,cm^{3}}{19.25\,g} \right )=3.17*10^{-23}\,cm^{3}\]
-
Calculate length of unit cell
\[V=l^{3}\]
\[l=\sqrt[3]{V}\]
\[l=\sqrt[3]{V}=\sqrt[3]{3.17*10^{-23}cm^{3}}=3.17*10^{-8}cm*\left ( \frac{10^{10}\AA }{100cm} \right )=3.17\AA=a\]
- Calculate radius of unit cell
\[b^{2}=a^{2}+a^{2}\]
\[b=\sqrt{2a^{2}}=\sqrt{2*(3.17)^{2}}=4.48\AA\]
\[c^{2}=a^{2}+b^{2}\]
\[c=\sqrt{a^{2}+b^{2}}=\sqrt{(3.17)^{2}+(4.48)^{2}}=5.49\AA\]
\[c=4r\]
\[r=\frac{c}{4}=\frac{5.49\AA }{4}=1.37\AA\]
Highlight here for hypothes.is annotations and indicate question number in your annotation.
How many atoms per unit cell are there in each of the following cubic lattice types?
|
|
simple cubic |
body-centered cubic |
face-centered cubic |
|
(a) |
8 |
6 |
12 |
|
(b) |
1 |
2 |
4 |
|
(c) |
1 |
2 |
6 |
|
(d) |
6 |
8 |
14 |
- Answer
-
b
Highlight here for hypothes.is annotations and indicate question number in your annotation.
10) Rank the following cubic cells in order of increased packing efficiency (least to most efficient)
a) primitive < body centered < face centered
b) face centered < primitive < body centered
c) primitive < body centered < face centered
d) face centered < body centered < primitive
- Answer
-
a) primitive < body centered < face centered
Highlight here for hypothes.is annotations and indicate question number in your annotation.
The number of unit cells that share an atom represented by the filled circle in the diagram below is:
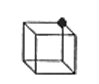
- Answer
-
d. 8
Ionic Solids
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Rank the following in order of increasing crystal lattice energy (the greater the energy the greater the bond dissociation energy)
a) Al2O3 < AlCl3 < BeCl2 < NaCl < NaF
b) Al2O3 < AlCl3 < BeCl2 < NaF < NaCl
c) NaCl < NaF < BeCl2 < AlCl3 < Al2O3
d) NaF < NaCl < BeCl2 < AlCl3 < Al2O3
- Answer
-
c) NaCl < NaF < BeCl2 < AlCl3 < Al2O3
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Bonding in Metals and Semiconductors
Network and Amorphous Solids
Phase Diagrams
Exercise \(\PageIndex{6a}\)
A substance under normal conditions would rather sublime than melt if _____.
- Its critical point occurs at a pressure above atmospheric pressure
- Its critical point occurs at a temperature above room temperature
- Its critical temperature is above its normal boiling point
- Its triple point occurs at a pressure above atmospheric pressure
- Its triple point occurs at a pressure below atmospheric pressure
- Answer
-
d. Its triple point occurs at a pressure above atmospheric pressure
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6b}\)
If a phase diagram has a solid-liquid phase boundary line that has a negative slope (leans to left) the substance,
- Can go from solid to liquid, within a small temperature range, via the application of pressure
- Cannot be liquefied above its triple point
- Cannot go from solid to liquid by application of pressure at any temperature
- Melts rather than sublimes under ordinary conditions
- Sublimes rather than melts under ordinary conditions
- Answer
-
a. Can go from solid to liquid, within a small temperature range, via the application of pressure
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6c}\)
The critical temperature, on a phase diagram, is _____.
- The temperature above which a gas cannot be liquefied
- The temperature at which all these states are in equilibrium
- The temperature below which a gas cannot be liquefied
- The temperature required to cause sublimation of a solid
- The temperature required to melt a solid
- Answer
-
a. The temperature above which a gas cannot be liquefied
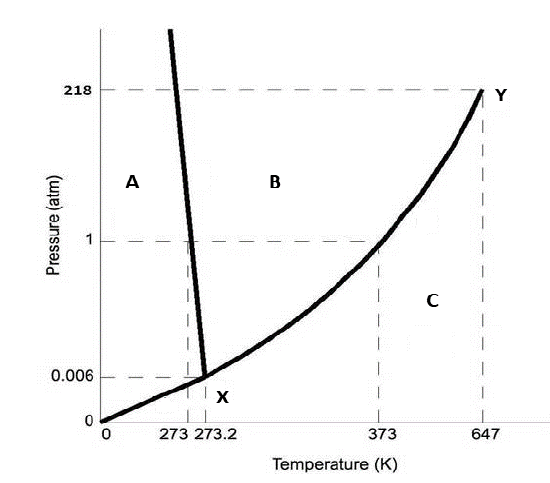
Figure 12.7.1: Use this figure to answer the following questions.
Exercise \(\PageIndex{6d}\)
The point X represents
- the critical point, where a solid, liquid and vapor can coexist
- The critical point where the two fluid phases cannot be distinguished
- The triple point, where a solid, liquid and vapor can coexist
- The triple point, where the fluid phases cannot be separated
- Answer
-
c. The triple point, where a solid, liquid and vapor can coexist
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6e}\)
The point Y in the figure represents
- the critical point, where a solid, liquid and vapor can coexist
- The critical point where the two fluid phases cannot be distinguished
- The triple point, where a solid, liquid and vapor can coexist
- The triple point, where the fluid phases cannot be separated
- Answer
-
b. The critical point where the two fluid phases cannot be distinguished
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6f}\)
Region A of the figure represents
- solid
- liquid
- vapor
- none of the above
- Answer
-
a. solid
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6g}\)
Region B of the figure represents
- solid
- liquid
- vapor
- none of the above
- Answer
-
b. liquid
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6.8}\)
Region C of the figure represents
- solid
- liquid
- vapor
- none of the above
- Answer
-
c. vapor
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6h}\)
The negative slope between regions A and B of figure 12.7.1 indicates:
- the solid is denser than the liquid
- the liquid is denser than the solid
- the vapor is denser than the liquid
- the vapor is denser than the solid
- Answer
-
b. the liquid is denser than the solid
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6i}\)
Figure 12.7.1 is consistent with a phase diagram for which compound
- carbon dioxide
- sodium
- water
- carbon dioxide and water
- Answer
-
c. water
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6j}\)
The compound in figure 12.7.1 sublimes at pressures:
- greater than deg O°C
- Pressures greater than 1.0 atm
- pressures between 0.0060 and 1.00 atm
- pressures less than 0.0060 atm
- Answer
-
d. pressures less than 0.0060 atm
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6k}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Moving left to right in region A represents
- freezing
- melting
- heating supercooled ice
- none of the above
- Answer
-
c. heating supercooled ice
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6l}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Adding heat to a substance in region A causes it to warm, what happens when you reach the line between region A & B?
- it boils
- it melts
- it freezes
- it continues to warm up
- Answer
-
b. it melts
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6m}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Moving left to right in region B represents
- melting
- boiling
- heating liquid water
- cooling liquid water
- Answer
-
c. heating liquid water
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6n}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Adding heat to a substance in region B causes it to warm, what happens when you reach the line between region B & C?
- it continues to warm
- it condenses
- it boils
- all of the above
- Answer
-
c. it boils
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6o}\)
Consider a 1 atm isobar for the compound in figure 12.7.1. Moving left to right in region C represents
- cooling water
- heating liquid water
- heating ice
- heating steam
- Answer
-
d. heating steam
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6p}\)
At what pressure can liquid, solid and gaseous water coexist?
- 218 atm
- 1.00 atm
- 0.0060 atm
- none of the above
- Answer
-
c. 0.0060 atm
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6q}\)
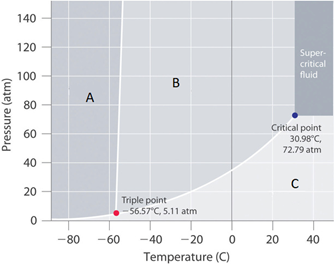
Consider a -50°C isotherm for the compound in Figure 12.7.2. Moving from region A to C represents
- Condensation then Freezing
- Freezing then Condensation
- Melting then Vaporizing
- Vaporizing then Melting
- Answer
-
c. Melting then Vaporizing
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6r}\)
Consider a 5 atm isobar for the compound in Figure 12.7.2. Moving from region C to A represents
- Condensation
- Deposition
- Sublimation
- Vaporization
- Answer
-
b. Deposition
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6s}\)
What phase would this compound be in if the pressure and temperature were at room conditions?
- Answer
-
This substance would be a gas
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6t}\)
The positive slope between regions A and B of figure 12.7.2 indicates:
- the solid is denser than the liquid
- the liquid is denser than the solid
- the vapor is denser than the liquid
- the vapor is denser than the solid
- Answer
-
a. the solid is denser than the liquid
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Exercise \(\PageIndex{6u}\)
Figure 12.7.2 is consistent with a phase diagram for which compound
- Carbon dioxide
- Carbon dioxide and water
- Sodium
- Water
- Answer
-
a. Carbon dioxide
Highlight here for hypothes.is annotations and indicate question number in your annotation.
Which of the following phase diagrams represents a substance that would sublime at atmospheric conditions?

- Answer
-
c.
Highlight here for hypothes.is annotations and indicate question number in your annotation.
What happens as a substance transitions from 3 to 5

- Answer
-
Freezes
Highlight here for hypothes.is annotations and indicate question number in your annotation.

