5.5: Enthalpy Changes of Chemical Reactions
- Page ID
- 158436
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Recognize that breaking bonds requires energy, and making new bonds releases energy
- Assess if the overall bond making and breaking process results in net energy input or output leading to overall exothermic or endothermic reactions
- Define standard reaction enthalpy and relate to stoichiometric coefficients in a a balanced chemical reaction
- Calculate the heat produced/consumed in chemical reaction based on amount of a reactants and products using standard reaction enthalpy and vice versa
"Flow of Energy" in a Reaction
When a reaction occurs, bonds in reactants break, the atoms rearrange and new bonds form as the products are created. If the bond energies of the formed bonds are different than the bond energies of the broken bonds, then the energy of the reactants is not equal to the energy of the products, and energy is either absorbed or released into the surroundings. There are a few things to note.
Breaking bonds always requires energy, and so it is endothermic.
Forming bonds is the opposite of breaking, and so it is exothermic.
Because enthalpy is a state function, we can energetically view a reaction as taking place in two steps. The first is the endothermic step of breaking the reactant bonds, and the second is the exothermic step of making the product bonds. The energy change of the reaction can be viewed as the sum of these two steps, and results in two possibilities.

Figure 5.5.1 Shows the two step way of viewing a reaction where the first step is the endothermic step involving the breaking of bonds, and the second is the exothermic step of making the bonds. This type of diagram is called an energy cycle because energy is conserved, and is valid because enthalpy is a state function.
Note, we are interested in ΔH(reaction) which only depends on the initial (reactant) and final (product) state, because enthalpy is a state function, and their difference is path independent. What we do know is that the first step required energy and the second released energy. In the Endothermic Reactions the first step is the larger, and so the products are higher in energy than the reactants. In exothermic reactions the first step is smaller, absorbing less energy than is released during the second step, and so the products are lower in energy than the reactants.
In the following figure (fig. 5.5.2) we are only focusing on the energy difference between the initial and final states
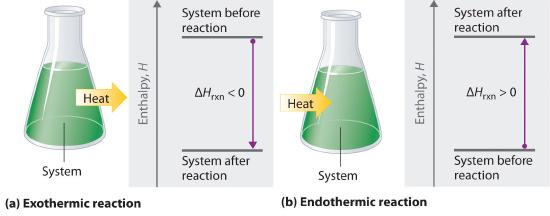
Figure 5.5.2 In this image we are only focusing on the reactant and product states, not the path. On the left side, the energy described the the enthalpy of reaction is released to the environment, and it warms the environment. On the right side, the system absorbs energy, cooling the environment.
Standard Reaction Enthalpy
In chapter 3 (section 3.1) we learned about Lavoisier's "Law of Conservation of Matter", and from this we learned to that a proper chemical equation must be balanced. In the next chapter 4, we applied stoichiometry to balanced equations and were able to predict reaction yields based on the complete consumption of the limiting reagent. In this section we are going to apply the First Law of Thermodynamics, the conservation of energy, to the balanced equation, and relate the energy of a chemical reaction to the stoichiometric coefficients of a reaction using the standard reaction enthalpy (ΔrH° ), the heat associated with the formation of a product in the pure form from the reactants in the pure form at standard temperature of 25°C (298K).
Endothermic Reactions
1. Endothermic Reactions: In endothermic reactions the heat is added to the system as reactants convert to products, and so the sign of ΔH is positive. Now the units of ΔHreaction often confuse students, as there are actually implicit units of mole in the denominator, where it is based on the stoichiometric coefficient of the chemical you are interested in. The easiest way to look at this is as an equivalence statement, where the enthalpy of reaction is related to the coefficient of each species.
Reactants + Heat → Products, ΔH>0
Example:
4CO2(g)+2H2O(l) → 2C2H2(g)+5O2(g) (ΔHreaction = 2599 kJ),
Looking at the energetics as a series of equivalence statements gives:
4 mole CO2 = 2559kJ
2 mole H2O = 2559kJ
2 mole C2H2 = 2559kJ
5 mole O2 = 2559kJ
So if 88.0 g C2H2 react, the energy released is
\[88.0g C_{2}H_{2} \left(\frac{ molC_{2}H_{2}}{16.024g}\right) \left(\frac{-2559kJ}{2molC_{2}H_{2}}\right) = -7,030kJ\]
Exothermic Reactions
2. Exothermic Reactions: In exdothermic reactions the heat is released from the system as reactants convert to products, and so the sign of ΔH is negative. Note, in the following example we looking at the reverse reaction of the prior example, and so the energy absorbed in the first is released in the second. That is, the only difference in the enthalpy of reaction is the sign, as the second equation is the reverse reaction of the first. This is an inportant principle and a consequence of the First Law of Thermodynamics. That is, if a reaction is endothermic in one direction, it is exothermic in the other, and the amount of energy absorbed in the endothermic direction is equal to the amount released in the exothermic direction.
Reactants + Heat → Products + Heat, ΔH<0
Example:
2C2H2(g)+5O2(g) → 4CO2(g)+2H2O(l) (ΔHreaction = -2599 kJ),
Looking at the energetics as a series of equivalence statements gives:
4 mole CO2 = 2559kJ
2 mole H2O = 2559kJ
2 mole C2H2 = 2559kJ
5 mole O2 = 2559kJ
If 88.0 g Oxygen react, how much energy is released?
\[88.0g O_{2}\left(\frac{ molO_{2}}{32.00g}\right) \left(\frac{2559kJ}{5molO_{2}}\right) = 1,410kJ\]
Exercise \(\PageIndex{1}\)
Consider the following reaction:
CO + 2 H2 → CH3OH + 128 kJ
A) Is the reaction endothermic or exothermic?
B) If 12.2 g of hydrogen gas are reacted _______________kJ are ______________(produced/consumed).
C) If 455 kJ of energy is produced, what was the mass of CO that was reacted?
- Answer
-
A) Exothermic. Since the heat is written on the right side of the equation, it can be thought of as heat produced or generated. So, it is an exothermic reaction. This reaction can also be written in this format, where the enthalpy is represented as having a negative value.

B) 387 kJ are produced:
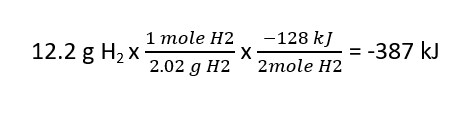
C) 99.6 grams of CO reacted:


