5.1: Energy
- Page ID
- 158432
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Define energy and work
- Define basic terms of thermodynamics like system and surroundings
- Describe the nature of energy transfers
- Compare and contrast exothermic and endothermic processes sign conventions associated with these process.
Introduction
In this chapter we will introduce thermodynamics, the science of energy and its transformations. Energy can be considered to be the capacity to do work or transfer heat.
Energy
Definition - Capacity to do work (w) or transfer heat (q)
In chapter 1 we introduced the concepts of kinetic and potential energy, and we will now take a deeper look at these. From a macroscopic perspective we sense objects as hot or cold as represented by the temperature, and recognize that heat will flow from hot to cold, but often mistake this to mean temperature is energy, which it is not. Thermal energy can be related to the random motion of molecules as they vibrate, rotate and translate in space.
Molecular Kinetic Energy
Translational Kinetic Energy:
\(E_{k} = \frac{1}{2}mv^{2}\), is the energy associated with the movement of a chemical entity's center of mass, where m is the mass of the chemical entity (molecule, atom or ion) and v is the velocity of it's center of mass. In the fluid phases (gas and liquids) there will be a distribution of translational energies as different molecules randomly move around each other.
Vibrational Potential Energy:
\(E_{k} = \frac{1}{2}kx^{2}\), is the energy associated with atom's vibrational motion, which can be modeled as a oscillating spring with k being Hooke's law constant and x is the displacement from the equilibrium location. That is, even the atoms in a solid, be it a covalent bond or ionic bond (crystal lattice) are static, and they vibrate back and forth around equilibrium positions like in a spring, with the the average distance being the "bond distance". Note, atoms oscillate because there are attractive and repulsive forces acting on them which change as their location changes. For example, in the diatomic hydrogen bond, the nuclei are accelerated towards each other if the attractive forces are greater than the repulsive, but as they get closer and closer, the nuclear/nuclear repulsion becomes stronger, and they start to be repulsed. As they move farther and farther apart, these forces become weaker, and the attractive forces once again begin to dominate, causing them to approach each other, with the resulting motion like the oscillation of a spring. Hooke's law equation actually describes the potential energy of the oscillator, when the distance is a maximum from the equilibrium, but as energy is conserved, this is equal to the kinetic energy that atoms feel in the midpoint of the oscillation. The hotter a substance the faster the vibrational frequency.
video 5.1.1. (4:38 min YouTube) from Open University. This is the first of 4 videos explaining how microwave ovens heat water. From 1:30 minutes to 3:40 minute an air puck attached to springs exemplifies oscillatory motion. In reality molecules are vibrating, rotating and translating.
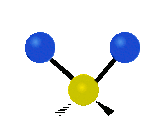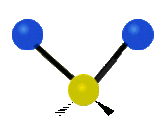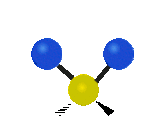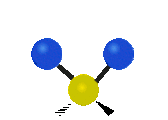


Fig. 5.1.1 Three fundamental vibrational modes for water, images from Wikipedia article on Electromagnetic absorption by water.
Rotational Kinetic Energy:
\(E_{\omega} = \frac{1}{2}I\omega^{2}\) , is the energy of a chemical entity associated with rotation, where \(\omega\) is the rotational frequency (how fast the molecule spins) and I is the moment of inertia (in a sense, it's resistance to spinning, the farther apart the hydrogen the greater I, the closer together, the smaller I. There can be multiple axis of rotation.
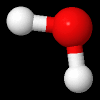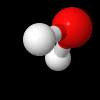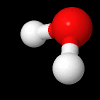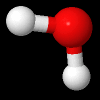
Fig. 5.1.2 Water molecule spinning along axis that bisects H-O-H angle, image from Wikipedia article on Electromagnetic absorption by water.
Video 5.1.2 (0:17 min YouTube) showing a figure skater reducing her moment of inertia by bringing her limbs close to her body with a resulting increase in rotational frequency to 308 RPMs. Not as she brings her arms and legs out she increases her moment of inertia, slowing down.
Molecular Potential Energy
Coulombic Energy is the energy between charged particles. There are both repulsive forces (between like charged particles) and attractive forces (between opposite charged particles).
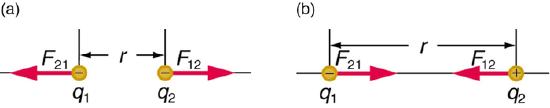
Fig. 5.1.3 image showing repulsion of like charges and attraction of opposite
Atomic Coulombic Energy:
The coulombic energy of attraction of the electron to the nucleus of an atom. These are weakest for outer shell electrons and strongest for core electrons.
Bond Coulombic Energies:
The energies of covalent and ionic bonds. In the case of ionic compounds they are the attractive and repulsive interactions of the various cations and anions. For covalent, they are the +/- attractions of electrons to nuclei of different atoms, but there are also repulsive interactions of the -/- electrons and +/+ nuclei. The The equilibrium distance is considered as the potential energy of the bond. As bonds are broken and formed during a chemical reaction energy thie potential energy may be released or absorbed from the surroundings which either gain or lose kinetic energy.
Nuclear Energy:
There are "strong" and "weak" forces holding the nucleous together and these will not be covered in this class.
Units of Energy
Joule
Work based definition of energy
This is the SI unit of energy and one Joule is a Newton meter (J = N.m), where a Newton is the SI unit of force. The Newton (named after Isaac Newton) comes from classical mechanics and Newton's second Law of motion, F = ma (mass times acceleration), and acceleration is the change in velocity per unit time, \(a=\frac{\Delta v}{\Delta t}\) and velocity is the vectorial change in displacement per unit time, \(v=\frac{\Delta x}{\Delta t}\) , where x is the displacement in a specific direction. So like any derived SI unit, the Joule can be described in terms of the SI base units, \(1J=1kg\frac{m^{2}}{sec^{2^{2}}}\)
Calorie
Heat based definition of energy
Historically, the calorie is the energy required to raise one gram of water one degree celsius (from 14.5oC to 15.5oC), but today the calorie is considered to be a depreciated unit, and according to NIST Special Publication 1038, "The International System of Units (SI)-Conversion Factors of General Use", the definition of the calorie is based on the Joule:
1Cal = 4.184 Joules
What is Work?
Work has many forms, of which mechanical(translational) work can be described as the energy used to displace an object a distance d when opposed against a force, like that of gravity.
W=Fd, where
- w is the work
- F is the opposing force
- d is the distance the object was displaced.
Note, there are other types of work, like the expansion of a gas and electric work (the work done to move a charged particle in an electric field). In this class we will mostly be interested in PV work, which can be visualized as the work done through the translational motion of molecules moving in three dimensional space. We will go into more details about PV work in Sec 5.4 when we discuss the First Law of Thermodynamics.
System and Surroundings*
Before we begin to keep track of energy changes, we must understand some basic terminology in thermodynamics. A thermodynamic system includes anything whose thermodynamic properties are of interest. It is embedded in its surroundings or environment; it can exchange heat with, and do work on, its environment through a boundary, which is the imagined wall that separates the system and the environment (Figure \(\PageIndex{4}\)). In reality, the immediate surroundings of the system are interacting with it directly and therefore have a much stronger influence on its behavior and properties. For example, if we are studying a car engine, the burning gasoline inside the cylinder of the engine is the thermodynamic system; the piston, exhaust system, radiator, and air outside form the surroundings of the system. The boundary then consists of the inner surfaces of the cylinder and piston.
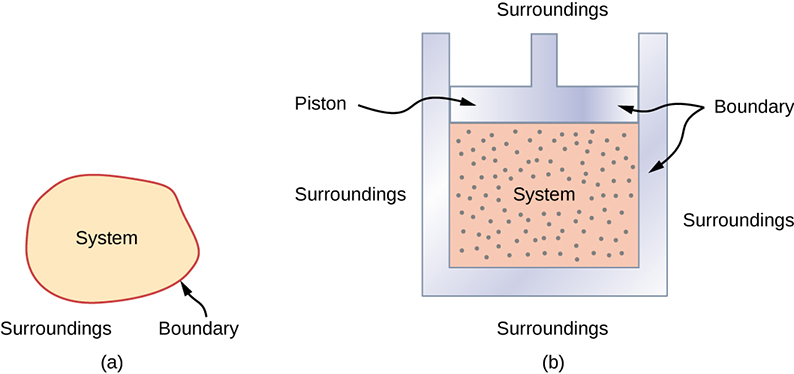
Figure \(\PageIndex{4}\): (a) A system, which can include any relevant process or value, is self-contained in an area. The surroundings may also have relevant information; however, the surroundings are important to study only if the situation is an open system. (b) The burning gasoline in the cylinder of a car engine is an example of a thermodynamic system
Normally, a system must have some interactions with its surroundings. A system is called an isolated or closed system if it is completely separated from its environment—for example, a gas that is surrounded by immovable and thermally insulating walls. In reality, a closed system does not exist unless the entire universe is treated as the system, or it is used as a model for an actual system that has minimal interactions with its environment. Most systems are known as an open system, which can exchange energy and/or matter with its surroundings (Figure \(\PageIndex{5}\)).
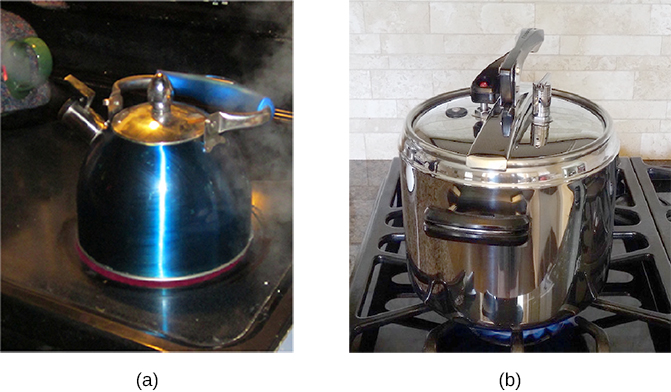
Figure \(\PageIndex{5}\): (a) This boiling tea kettle is an open thermodynamic system. It transfers heat and matter (steam) to its surroundings. (b) A pressure cooker is a good approximation to a closed system. A little steam escapes through the top valve to prevent explosion. (credit a: modification of work by Gina Hamilton)
Exothermic and Endothermic Processes**
When physical or chemical changes occur, they are generally accompanied by a transfer of energy. The law of conservation of energy states that in any physical or chemical process, energy is neither created nor destroyed. In other words, the entire energy in the universe is conserved. In practical terms for a laboratory chemist, the system is the particular chemicals being reacted, while the surroundings is the immediate vicinity within the room. During most processes, energy is exchanged between the system and the surroundings. If the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings.
A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The quantity of heat for a process is represented by the letter \(q\). The sign of \(q\) for an endothermic process is positive because the system is gaining heat. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings is gaining heat from the system, the temperature of the surroundings increases. The sign of \(q\) for an exothermic process is negative because the system is losing heat.
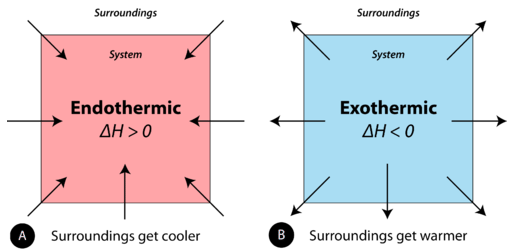
Figure \(\PageIndex{6}\): (A) Endothermic reaction. (B) Exothermic reaction.
Contributors:
- Robert E. Belford (UA of Little Rock)
- Ronia Kattoum (UA of Little Rock)
- *System and Surroundings Original Article
- **Exothermic and Endothermic Processes Original Article

