4.4: Chemical Equations and Quantitative Analysis
- Page ID
- 158426
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Learning Objectives
- Differentiate between quantitative and qualitative analysis
- Determine the amount of unknown solute in a solution using the gravimetric analysis technique
- Determine the empirical formula of a hydrocarbon or an organic compound using the combustion analysis technique
Quantitative Analysis is a branch of analytical chemistry where you determine the "quantity" of an unknown, which is often contrasted to qualitative analysis, which seeks to identify the identity of unknown. There are many types of quantitative techniques that are based on stoichometry, and many of these deal with solutions, and will be covered in the next several sections of this chapter. In this section, we will focus on two types, gravimetric analysis and combustion analysis.
Gravimetric Analysis
Gravimetric analysis is a technique used to identify the quantity of a solute in solution (or mixture such as mineral) by making it the limiting reagent of precipitation reaction, weighting the mass of the resulting precipitate that forms, and then using the stoichiometry of the precipitation reaction to "back solve" for the quantity of the unknown. This technique takes advantage of the solubility rules, and the strategy is to use a double displacement reaction where where one of the products is soluble, while the other forms a precipitate.
For example, if you had a solution of barium chloride, you do not know the mass of barium chloride (solute) there is in the solution. If you wanted to identify the moles of soluble barium chloride in a solution, you could add excess sodium sulfate, the balanced molecular equations would be:
BaCl2(aq) + Na2SO4(aq) --> BaSO4(s) + 2NaCl(aq)
(limiting) (excess)
By adding excess sodium sulfate, you can force all the barium to form a precipitate, which can be filtered, dried, and weighed. Once the mass of precipitate is known, the moles barium sulfate can be calculated. From that, the moles (or mass) of the barium chloride can be determined. For this to work, the unknown must be the limiting reagent.
Quantitative Analysis of Lead(II) Nitrate.
Lead was found in the drinking water at Flint Michigan, and one way of removing it is through the double displacement reaction with phosphate, to create lead(II) phosphate, which is an insoluble salt. Interestingly, this forms a protective coating in the pipe that prevents further corrosion. In fact, the city of Flint had removed phosphate from the treatment process, as it also prevents corrosion of iron, and that is when the lead started to dissolve in the first place, as described in the Chemical and Engineering News article of the American Chemical Society.
What is the molar concentration of Pb+2 in contaminated water if 14.06 grams of Pb3(PO4)2(s) precipitates out of 1.00 liter of a lead(II)chloride solution
4:31 minute youtube determing lead(II) ion concentration in 1.00L of lead(II) chloride solution.VIRTUAL LAB
Combustion Analysis
This is a type of quantitative analysis that can be used to determine the empirical formula of an unknown organic compound consisting of carbon, hydrogen and oxygen. In combustion analysis, a sample of known mass is burned in excess oxygen. It is then passed through H2O and CO2 absorbers that collect the hydrogen(in the form of H2O) and carbon(in the form of CO2) that were in the original sample. Since we know the mass percent of hydrogen in water (2.01/18.01 or 11.2%) and the mass percent of carbon in carbon dioxide (12.01/44.01 or 27.3%) we can identify their masses from the water and carbon dioxide produced. Once those masses are known, the mass of the oxygen can be determined by subtracting the mass of the carbon and hydrogen from the original sample mass. This helps us determine the empirical formula, similar to what we did in chapter 2.
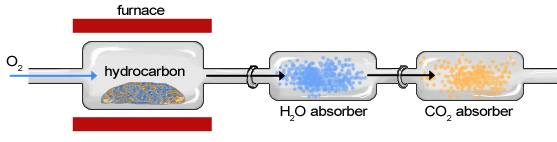
Figure 4.4.1
Combustion Analysis of Acidum Formicum, Formic Acid
In 1670 John Wray published in the Philosophical Transactions of the Royal Society of London his observations and experiments dealing with the "Juyce to be Found in Ants" .doi:10.1098/rstl.1670.0052
Formic acid is obtained through the distillation of Formica Rufa.
What is the empirical formula of formic acid(assuming the compound is made up of carbon, hydrogen, and oxygen) if 2.4527 g sample yielded 0.9696g water and 2.3482g carbon dioxide.?
5:27 min YouTube describing the combustion analysis of formic acid.
Exercise \(\PageIndex{1}\)
After burning 1.333 g of a hydrocarbon in a combustion analysis apparatus, 1.410 g of H2O
and 4.305 g of CO2 were produced. Separately, the molar mass of this hydrocarbon was found to be 204.35 g/mol. Calculate the empirical and molecular formulas of this hydrocarbon.
- Answer
-
Note: a hydrocarbon consists of only carbon and hydrogen.

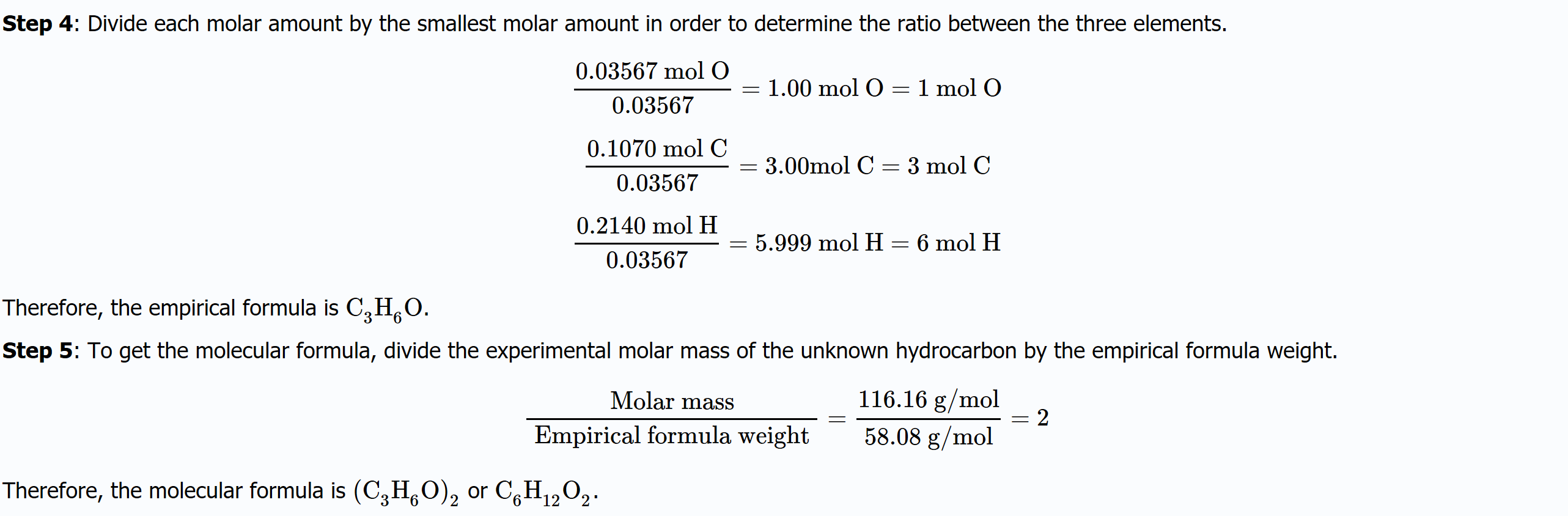
Exercise \(\PageIndex{2}\)
A 2.0714 g sample containing carbon, hydrogen, and oxygen was burned in a combustion analysis apparatus; 1.928 g of H2O and 4.709 g of
CO2 were produced. Separately, the molar mass of the sample was found to be 116.16 g/mol. Determine the empirical formula, molecular formula, and identity of the sample.
- Answer
-

Contributors:
- Robert Belford (UA of Little Rock)
- Modified by Ronia Kattoum (UA of Little Rock)
- Link to Original source for Exercise 1 and 2:

