2.4: The Periodic Table
- Page ID
- 158406
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning objectives
- Describe the evolution of the periodic table
- Memorize terminology used to describe the periodic table (periods, groups, group numbers, and group names).
- Locate elements on the periodic table and classify them as metals, nonmetals, metalloids
- Infer likely properties of elements based on their position on the periodic table.
Mendeleev’s Periodic Table
Mendeleev, who first published his periodic table in 1869 (Figure \(\PageIndex{1}\)), is usually credited with the origin of the modern periodic table. The key difference between his arrangement of the elements and that of Meyer and others is that Mendeleev did not assume that all the elements had been discovered (actually, only about two-thirds of the naturally occurring elements were known at the time). Instead, he deliberately left blanks in his table at atomic masses 44, 68, 72, and 100, in the expectation that elements with those atomic masses would be discovered. Those blanks correspond to the elements we now know as scandium, gallium, germanium, and technetium.
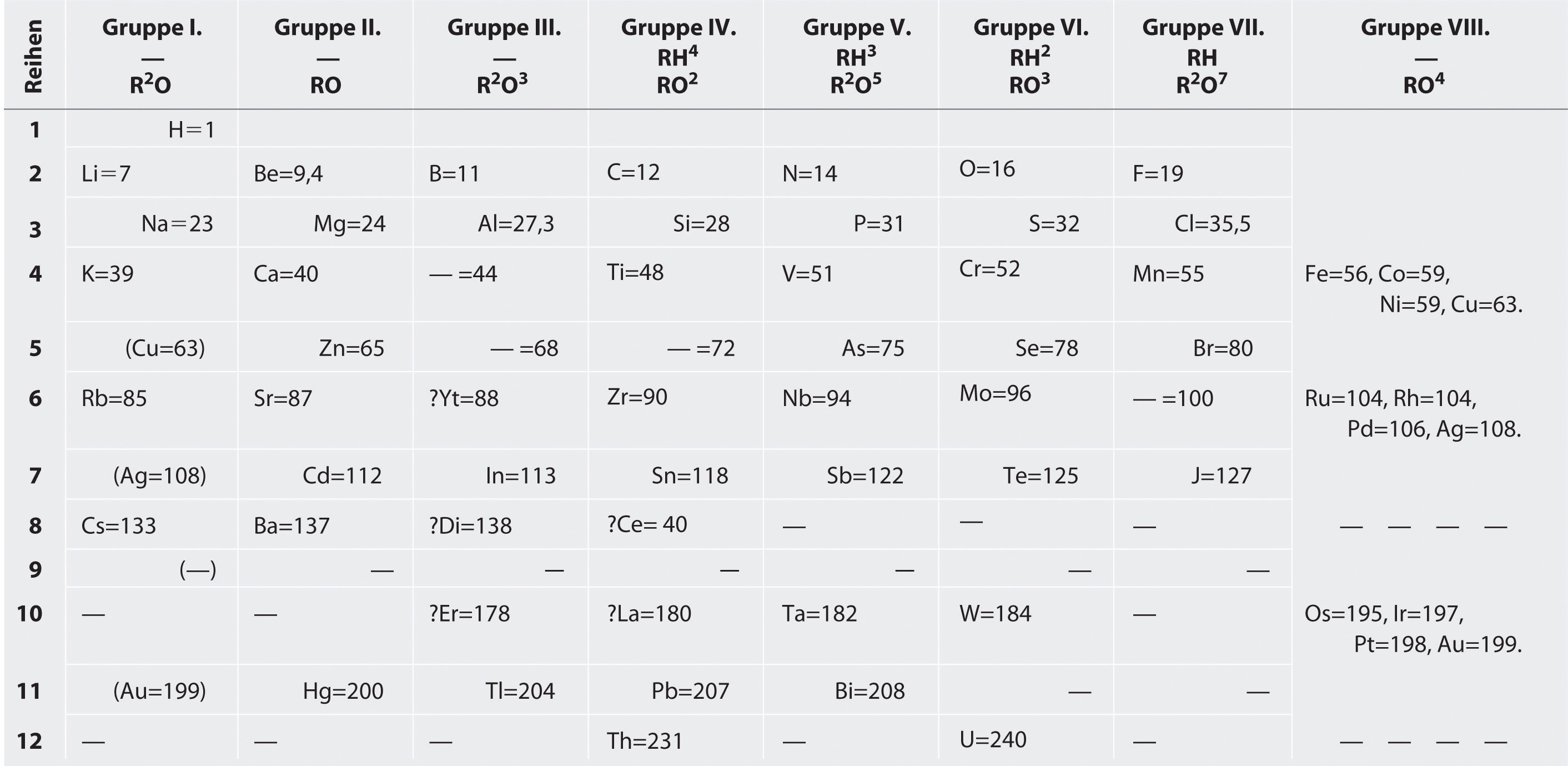
Figure \(\PageIndex{1}\) Mendeleev’s Periodic Table, as Published in the German Journal Annalen der Chemie und Pharmacie in 1872. The column headings “Reihen” and “Gruppe” are German for “row” and “group.” Formulas indicate the type of compounds formed by each group, with “R” standing for “any element” and superscripts used where we now use subscripts. Atomic masses are shown after equal signs and increase across each row from left to right.
The groups in Mendeleev's table are determined by how many oxygen or hydrogen atoms are needed to form compounds with each element. For example, in Group I, two atoms of hydrogen, lithium, Li, sodium, Na, and potassium form compounds with one atom of oxygen. In Group VII, one atom of fluorine, F, chlorine, Cl, and bromine, Br, react with one atom of hydrogen. Notice how this approach has trouble with the transition metals. Until roughly 1960, a rectangular table developed from Mendeleev's table and based on reactivity was standard at the front of chemistry lecture halls.
The most convincing evidence in support of Mendeleev’s arrangement of the elements was the discovery of two previously unknown elements whose properties closely corresponded with his predictions (Figure \(\PageIndex{1}\)). Two of the blanks Mendeleev had left in his original table were below aluminum and silicon, awaiting the discovery of two as-yet-unknown elements, eka-aluminum and eka-silicon (from the Sanskrit eka, meaning “one,” as in “one beyond aluminum”). The observed properties of gallium and germanium matched those of eka-aluminum and eka-silicon so well that once they were discovered, Mendeleev’s periodic table rapidly gained acceptance.
The Modern Periodic Table
Since Mendeleev's development of the first periodic table and various scientists working to fill in the gaps, the modern periodic table now has 118 known elements. You may click on any element in the following periodic table for every known detail about every element that has been discovered or synthesized.
Classification of the Periodic Table
If you tried to click on any of the above elements, the details of each element may seem overwhelming and it may seem incredibly difficult to find patterns. However, the beauty of the periodic table is found in it's periodicity, repeating patterns that help us predict physical and chemical properties of various elements.
Before we delve deep into classification, we must identify some terminology. The periodic table is split into 18 columns that are known as groups or families ) Groups can be numbered as 1-18 (Figure \(\PageIndex{2}\) ) or they can be numbered 1-8 followed by "A" or "B" (Figure \(\PageIndex{3}\)). The "A" designation represents the main group elements (Columns 1-2, and Columns 13-18) The "B" designation represents transition elements (Columns 3-12). Either group numbering system is acceptable, but we will see later that there is a benefit for knowing the group numbers with the "A" and "B" designation. In addition, some of the individual groups have a particular group name. For example, Group 17 or 7A elements are also known as the halogens. Group 2 or 2A elements are known as the alkaline group metals. Group names are commonly used by chemists and are worth knowing. But, most importantly, elements in the same groups tend to have similar chemical and physical properties. We will also discuss the reason behind such patterns later in this text.
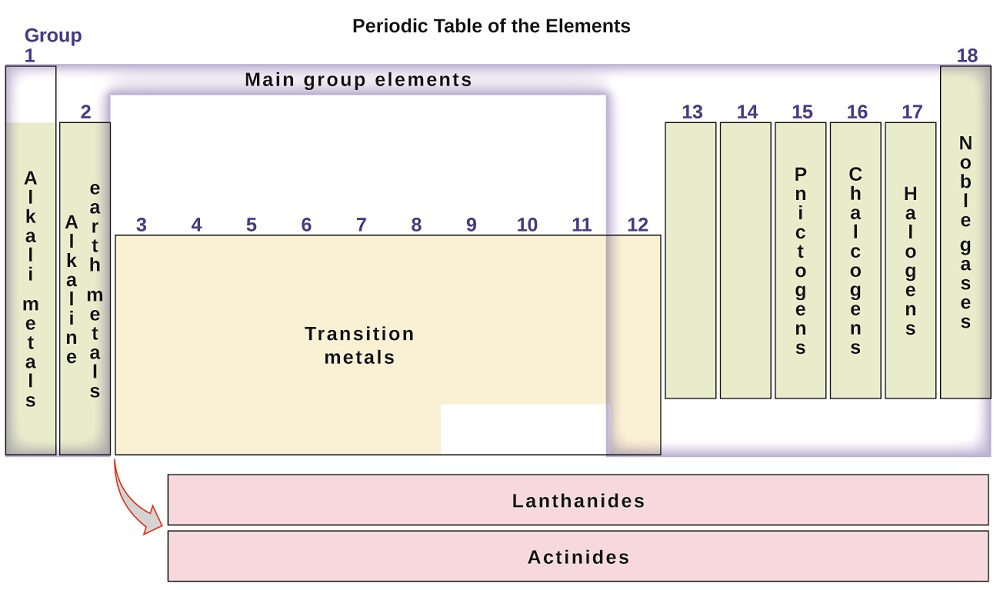
Figure \(\PageIndex{2}\) Terminology of Periodic Table
Figure \(\PageIndex{3}\) Metals/Nonmetals/Metalloids(Semimetals)
The periodic table can also be split into seven rows, known as periods (Figure \(\PageIndex{4}\)). At first glance, it may seem like the two lone rows at the bottom of the period table, known as the actinide and lanthanide series should be periods 8 and 9. However, if you look carefully, these two row should actually be wedged in the 6th and 7th period, respectfully, in order to follow the order of increasing atomic number. Those rows are kept at the bottom to decrease the width of the periodic table and are also known as the inner transition metals.
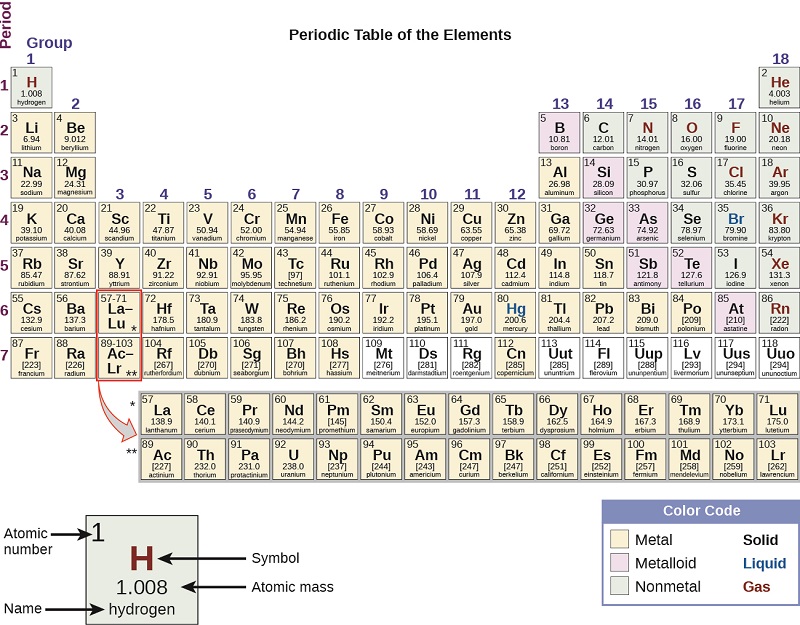
Figure \(\PageIndex{4}\)- The periodic table is split into seven periods(rows) with the inner transition metals shown separately on the bottom to decrease the width of the periodic table. It can also be broadly divided into metals, nonmetals, and metalloids(semimetals). The states of the elements is also indicated at room temperature.
In addition to assigning groups names and numbers, the periodic table is often broken into a classification of three types of elements; metals, nonmetals, and metalloids ( semi-metals). (Figure \(\PageIndex{3}\) and Figure \(\PageIndex{4}\)). If you start at boron and draw "stairs" down to astatine, the seven elements that are "on" are the metalloids or semi-metals. Everything to the left of the "stairs" with the exception of hydrogen is a metal. Everything to the right of the "stairs", in addition to hydrogen, is a nonmetal. This is essential for describing common physical properties for each of the three categories.
Physical Properties of Metals: Note, these can be related to the fact that electrons in metallic bonds are loosely bond between multiple core atoms and freely flow from one atom to another.
- Conduction (heat and electricity)
- Malleability (can be hammered into thin sheets)
- Ductility (can be pulled into wires)
- Lustrous (Shiny appearance)
Physical Properties of Non-Metals: Note, nonmetals are held together by directional covalent bonds of shared electrons, and this relates to their properties.
- Poor Conductors
- Nonlusterous
- Nonmalleable
- Brittle
- Brightly colored
Physical Properties of Metalloids (Semi-Metals):
They have both properties of metals and non-metals. One of the most important physical properties of metalloids is their semi-conductive properties.
Natural States of Atoms
Liquids: Hg, Br2, ( Ga, mp=85.58oF; Cs, mp=83.3oF; Fr, mp=80.6oF and Rb, mp=103.1 oF)
Gasses: Noble Gasses and Lighter diatomics, H2, N2, O2, F2, & Cl2
Solids: All other elements
Exercise \(\PageIndex{1}\)
What is the group number for oxygen?
A) 16
B) 6
C) 16A
D) Both A and B
- Answer
-
A only. B is incorrect because it does not have a “A” following the 6. So, it is either 16 or 6A. Group 6 refers to the chromium group.
Exercise \(\PageIndex{2}\)
What is true regarding the classification of the following silicon? (select all that apply)
A) group 4A
B) metalloid
C) main group element
D) period 14
E) nonmetal
- Answer
-
A, B, and C are all correct. D is incorrect. The period (row) is 3. The group is 13. E is incorrect because silicon sits on the stairs and is one of the metalloids.
Exercise \(\PageIndex{3}\)
What alkali metal is found in period 4?
A) carbon
B) potassium
C) calcium
D) Titanium
- Answer
-
B) Potassium is in Group (Column) 1A and is in the 4th period (row).
Exercise \(\PageIndex{4}\)
Which of the following elements will most likely have similar chemical properties to fluorine?
A) neon
B) nitrogen
C) iodine
D) sulfur
- Answer
-
C) iodine. Elements in the same group have similar chemical properties, not the same periods.
Contributors
- Bob Belford
- Ronia Kattoum (UA of Little Rock)

