10.1 Gas Properties
- Page ID
- 158470
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
In section 1.3 we introduced the phases of matter and in section 5.3 we covered phase transitions from a thermodynamic perspective. In this chapter we will take a deeper look into the gas phase. We will start with covering basic gas properties and then learn several "equations of state", which are mathematical equations that relate measurable variables that describe the quantity of particles (n), volume, pressure and temperature of a gas. We will apply these to gas phase stoichiometery, describe multi-component gases as homogenous mixtures (solutions), cover the kinetic molecular theory which described an ideal gas and how real gasses can deviate from it. We will also look at gasses as dispersed particulate systems and cover properties like effusion and diffusion.
What are the characteristics of the gaseous state?
- Fluid - A gas will take the shape of a container it fills. There is zero viscosity (resistance to flow) and it fills the entire container.
- Compressible - The density of a gas changes as its volume changes. Thus, unlike incompressible solids or liquids, density alone can not be used to help identify a gas. That is, water (l) has a density of 1g/ml at normal temperature and pressure, and so that can be used to help identify a substance as water. But because a gas fills a container, its density depends on the size of the container.
- Forms Solutions - Gas forms homogenous mixtures. Note, for liquid phase solutions we often identify a solvent and a solute, where the solute is dissolved in the solvent to form a solution. We usually consider the solvent to be the substance in greatest quantity, and an aqueous solution is one where water is the solvent. This is because the "space" of matter is being occupied by the liquid particles (which is why they are incompressible). In the gas phase, most of the "space" of matter is the "void" (which is why gasses are compressible), and so it is not normal to consider gas phase solutions to have a solute and solvent. Instead we use the concept of mole fraction to identify the relative proportions of different species in a gas mixture.
How do we measure the quantity of particles in a gaseous state?
State Variables - we need an equation of state that relates measurable state variables to the number of gas particles. The Ideal Gas Law (PV=nRT) is an equation of state that relates the measurable state variables of Pressure, Temperature and Volume to the number of particles. There are other equations of state and at the end of the chapter we will look at a few,
PV=nRT
P=Pressure, V=Volume, n=moles, T=Temperature (Kelving)
R=0.08206(l-atm/mol-K)
Pressure
Pressure is defined as force per unit area, \(P=\frac{F}{A}\) and the SI unit is the Pascal, \(Pa=\frac{1N}{m^{2}}=\frac{1kg}{m\cdot s^{2}}\). This pressure of a gas can be understood by visualizing gas particles in constant motion, as exemplified in fig. 10.1.1

Fig. 10.1.1: Gas particles in a sealed container. As the gas particles move they eventually hit the surface and impart a force on the surface as they change their direction of motion (red particles). The pressure is this force per unit area due to these collisions, which in SI units is the Pa (1N/m2).
It is more common to see pressure described in atm or torr (mm Hg), which are the units we will predominantly use in this class, and have macroscopic definitions that are more easily measured. Table 10.1.1 shows the common units of pressure, and their conversion factors. Note, because this table is all set to one atmosphere, you can go directly between the other units (14.7 lb/in2 = 1.10x105 Pa = 760 torr...)
| 1 atm = | 760 torr |
| 1 atm = | 760 mm Hg |
| 1 atm = | 14.7 lb/in2 |
| 1 atm = | 1.01325x105 Pa |
Table 10.1.1: Common units of pressure and conversion factors. UALR students are required to memorize the conversion factors in red.
Atmosphere:
One atmosphere of pressure is roughly the pressure felt at sea level by earth's atmosphere, as is shown in figure 10.1.2. Note, this is not an easily reproducible value because the force felt on the earth changes over both your location and time. This YouTube from the National Naval Aviation Museum does a good job of showing how the air pressure changes as a function of altitude. That is, at the top of Mt Everest (about 5.5 miles high) there is a smaller column of air above you and so the pressure is less than at sea level, while in the Salton Sea CA (-228 ft) the pressure is higher than at sea level.
But also, because the atmosphere of the earth is really a mixture of gasses (mostly nitrogen and oxygen), with many species like water vapor, carbon dioxide, and air pollutants varying over time, the mass of the column changes over time. This is why the weatherman reports barometric pressure, because on humid days the increased number of vaporized water molecules causes the pressure to go up (and thus the chance of rain), while on arid days the pressure goes down, because there are fewer water molecules. So although the concept of an atmosphere of pressure is easy to understand, and it is important because many reactions that occur on the surface of the earth occur at around one atmosphere of pressure, its variance over time and location makes it hard to develop a scale on.

fig. 10.1.2: This image illustrates the concept of an atmosphere of pressure, which is the pressure felt at sea level due to the column of earth's atmosphere.
Torr and the Barometer:
The torr or mm Hg is a reproducible definition of pressure based on the height of a column of mercury, and can be used to make an instrument to measure atmospheric pressure, the barometer. Using the conversion factor of 1 atm = 760 mm Hg, we could calibrate the barometer in units of atm. This is illustrated in figure 10.1.3, where the height of the mercury column is dependent on the atmospheric pressure. If the atmospheric pressure is one atm the column is exactly at 760 mm Hg (760 torr). On a humid day the pressure outside goes up and the system is no longer at equilibrium, which forces the mercury up the tube (greater than 760 torr) until they balance out. On an arid day it lowers until they balance out.

Fig. 10.1.3: Mercury barometer: The above device can be used to measure atmospheric pressure by measuring the height of the mercury column, and noting that 760 mm Hg equals 1 atm.
The following video from the North Carolina School of Science and Mathematics (NCSSM) shows how to make a mercury barometer.
Video 10.1.1 (1:35 min) YouTube from NCSSM on how to make a barometer.
Why do you think the column of mercury is 744 mm Hg in the above youtube from NCSSM?
Obviously NCSSM is above sea level, but on closer look, it is in Durham NC, which is only 400 feet above sea level, and so it was also probably an arid day.
How could you measure the pressure of a gas in a sealed container?
A manometer is similar to a barometer, but allows you to measure the pressure of a gas in a sealed container.
Manometer
The manometer is the device that can measure the pressure of a gas in a closed container. There are two types of manometers, open ended and closed ended. Figure 10.1.4 shows the two basic types of manometers. The principle is that if the gas pressure on the left and right side of the youtube are not equal, the system will adjust until they are equal.
Fig. 10.1.4 Closed and open ended manometers.
Closed Barometer: In the closed barometer you are pushing the gas against a column of mercury where the opposite end is in a vacuum, and so the column of of fluid goes up until the pressure due to the column equals the pressure of the gas, and if the fluid is mercury, this is the pressure in mm Hg (torr).
Open Barometer. For the open barometer there are three options. The gas is less than, equal to, or greater than the atmosphere. If the gas pressure equals atmospheric pressure there is no difference in the heights of the columns.
- Gas Pressure < Atmospheric Pressure
Pgas + h = Patm
The middle manometer in figure 10.1.4 shows this case and since the atm pressure is greater it moves the mercury in towards the gas container until the forces cancel out. - Gas Pressure > Atmospheric Pressure
Pgas = Patm + h
The right manometer in figure 10.1.4 shows the case where the gas pressure is greater, and so it pushes the mercury back against to atmosphere until the forces balance out.
Note: We are treating these for mercury manometers as the height of the column in mm Hg is the definition of a unit of pressure. The same principle works for any incompressible fluid, where the pressure in Pascel is given by \(\rho\)gh, where \(\rho\) is the density, g is the gravitational constant (9.81 m/s2) and h is the height of the column.
Exercise: Show that 760 mm Hg = 101.5 x 105 Pa
Solution: For mercury, \(\rho\) = 13.6g/cm3 and P=\(\rho\)gh. Pleae note, physics is not a prerequisite for this course and we have not gone over gravitational forces, but you should be able to do this problem based on two things. First you were given the equation and so should be able to substitute in the values, and second, you know the Pa is the SI unit, and can be expressed in terms of the 7 SI base units, which for this problem were the kg (mass), m (length) and time (s). So all you have to do is convert the units to the SI base units, and you have the value in Pa.
\[P=\rho gh=13.6\frac{g}{cm^{3}}\left (9.81 \frac{m}{s^{2}} \right )760mm\left ( \frac{kg}{1000g} \right )\frac{(100cm)^{3}}{m^{3}}\left ( \frac{1m}{1000mm} \right )=1.0139x10^{5}\frac{kg}{m\cdot s^{2}}=1.0139x10^{5}P\]
This Page is Under Construction
The content on this page has been temporarily borrowed from:
Skills to Develop
- To describe the characteristics of a gas.
The three common phases (or states) of matter are gases, liquids, and solids. Gases have the lowest density of the three, are highly compressible, and completely fill any container in which they are placed. Gases behave this way because their intermolecular forces are relatively weak, so their molecules are constantly moving independently of the other molecules present. Solids, in contrast, are relatively dense, rigid, and incompressible because their intermolecular forces are so strong that the molecules are essentially locked in place. Liquids are relatively dense and incompressible, like solids, but they flow readily to adapt to the shape of their containers, like gases. We can therefore conclude that the sum of the intermolecular forces in liquids are between those of gases and solids. Figure 6.1.1 compares the three states of matter and illustrates the differences at the molecular level.
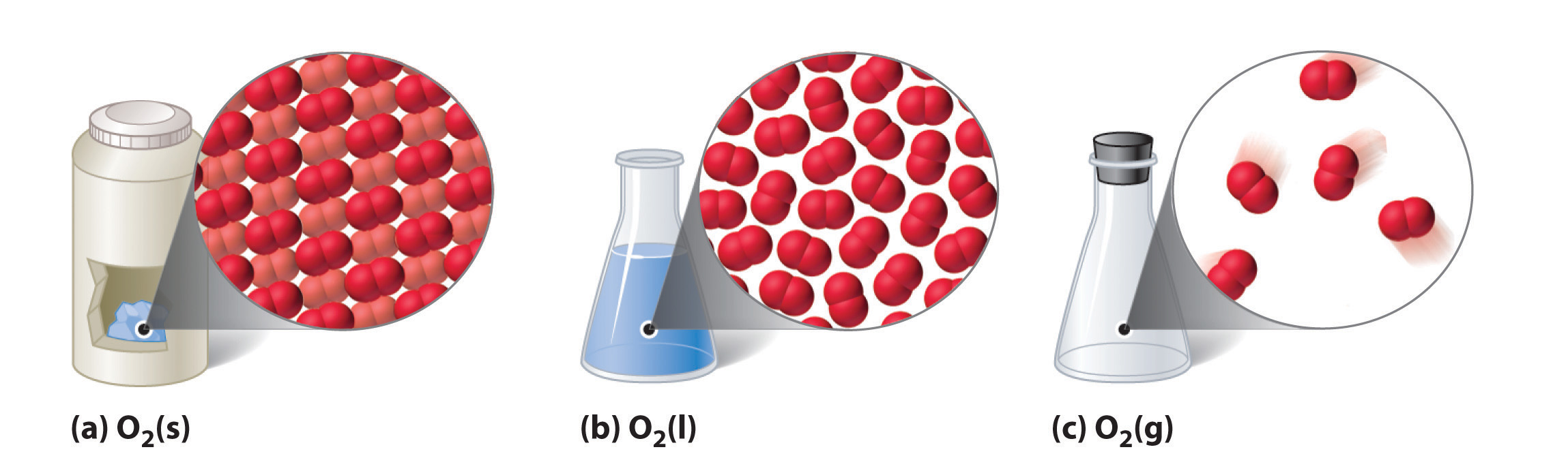
Figure 6.1.1: A Diatomic Substance (O2) in the Solid, Liquid, and Gaseous States: (a) Solid O2 has a fixed volume and shape, and the molecules are packed tightly together. (b) Liquid O2 conforms to the shape of its container but has a fixed volume; it contains relatively densely packed molecules. (c) Gaseous O2 fills its container completely—regardless of the container’s size or shape—and consists of widely separated molecules.
The state of a given substance depends strongly on conditions. For example, H2O is commonly found in all three states: solid ice, liquid water, and water vapor (its gaseous form). Under most conditions, we encounter water as the liquid that is essential for life; we drink it, cook with it, and bathe in it. When the temperature is cold enough to transform the liquid to ice, we can ski or skate on it, pack it into a snowball or snow cone, and even build dwellings with it. Water vapor (the term vapor refers to the gaseous form of a substance that is a liquid or a solid under normal conditions so nitrogen (N2) and oxygen (O2) are referred to as gases, but gaseous water in the atmosphere is called water vapor) is a component of the air we breathe, and it is produced whenever we heat water for cooking food or making coffee or tea. Water vapor at temperatures greater than 100°C is called steam. Steam is used to drive large machinery, including turbines that generate electricity. The properties of the three states of water are summarized in Table 6.1.1.
| Temperature | State | Density (g/cm3) |
|---|---|---|
| ≤0°C | solid (ice) | 0.9167 (at 0.0°C) |
| 0°C–100°C | liquid (water) | 0.9997 (at 4.0°C) |
| ≥100°C | vapor (steam) | 0.005476 (at 127°C) |
The geometric structure and the physical and chemical properties of atoms, ions, and molecules usually do not depend on their physical state; the individual water molecules in ice, liquid water, and steam, for example, are all identical. In contrast, the macroscopic properties of a substance depend strongly on its physical state, which is determined by intermolecular forces and conditions such as temperature and pressure.
Figure 6.1.2 shows the locations in the periodic table of those elements that are commonly found in the gaseous, liquid, and solid states. Except for hydrogen, the elements that occur naturally as gases are on the right side of the periodic table. Of these, all the noble gases (group 18) are monatomic gases, whereas the other gaseous elements are diatomic molecules (H2, N2, O2, F2, and Cl2). Oxygen can also form a second allotrope, the highly reactive triatomic molecule ozone (O3), which is also a gas. In contrast, bromine (as Br2) and mercury (Hg) are liquids under normal conditions (25°C and 1.0 atm, commonly referred to as “room temperature and pressure”). Gallium (Ga), which melts at only 29.76°C, can be converted to a liquid simply by holding a container of it in your hand or keeping it in a non-air-conditioned room on a hot summer day. The rest of the elements are all solids under normal conditions.
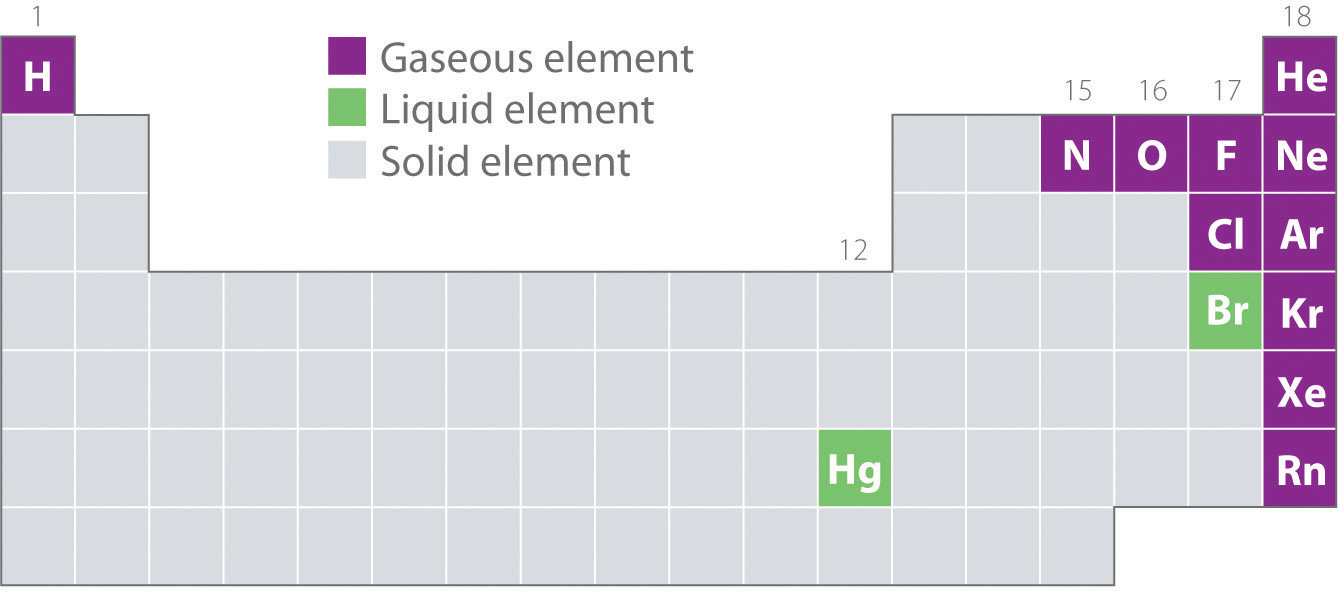
Figure 6.1.2: Elements That Occur Naturally as Gases, Liquids, and Solids at 25°C and 1 atm. The noble gases and mercury occur as monatomic species, whereas all other gases and bromine are diatomic molecules.
All of the gaseous elements (other than the monatomic noble gases) are molecules. Within the same group (1, 15, 16 and 17), the lightest elements are gases. All gaseous substances are characterized by weak interactions between the constituent molecules or atoms.
Summary
Bulk matter can exist in three states: gas, liquid, and solid. Gases have the lowest density of the three, are highly compressible, and fill their containers completely. Elements that exist as gases at room temperature and pressure are clustered on the right side of the periodic table; they occur as either monatomic gases (the noble gases) or diatomic molecules (some halogens, N2, O2).

