8.7: Bond Polarity and Electronegativity
- Page ID
- 169775
Bond Polarity and Electronegativity
Exercise \(\PageIndex{1}\)
Which of the following bonds would be the most polar?
a. N--C b. N--Si c. N--P d. N--Al e. N--Ga
- Answer
-
e. N--Ga
Exercise \(\PageIndex{2}\)
Which of the following compounds has polar covalent bonds: CCl4, Cl2, HCl, and KCl?
- Answer
-
CCl4 and HCl
Exercise \(\PageIndex{3}\)
Electronegativity is a measure of _____.
- the ability of a substance to conduct electricity
- the charge on a polyatomic cation
- the charge on a polyatomic anion
- the ability of an atom in a molecule to attract electrons to itself
- the oxidation number of an atom in a molecule or polyatomic anion
- Answer
-
d. the ability of an atom in a molecule to attract electrons to itself
Exercise \(\PageIndex{4}\)
Three nonequivalent Lewis structures for carbonyl sulfide, SCO, are given below. Use the concepts of formal charge and electronegativity to choose the structure that is the best representation.
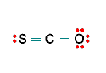
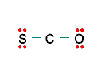
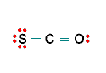
A B C
- Structure A, because all the formal charges equal 0
- Structure B, because all the formal charges equal 0
- Structure C, because all the formal charges equal 0.
- Structure A, because the negative formal charge resides on the most electronegative atom
- Structure C, because the negative formal charge resides on the most electronegative atom
- Answer
-
b. Structure B, because all the formal charges equal 0
Exercise \(\PageIndex{5}\)
Choose which central atom in the following molecules is most electronegative.
a. PH3 b. CH4 c. H2S d. H2O e. NH3
- Answer
-
d. H2O
Exercise \(\PageIndex{6}\)
Rank the following covalent bonds in order of decreasing polarity: C-H, N-H, O-H, and F-H.
- Answer
-
F-H > O-H > N-H > C-H
Exercise \(\PageIndex{7}\)
Atoms having equal or nearly equal electronegativities are expected to form
- no bonds
- polar covalent bonds
- nonpolar covalent bonds
- ionic bonds
- covalent bonds
- Answer
-
c. nonpolar covalent bonds

