8: Bonding and Molecular Structure
- Page ID
- 169768
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ionic or Covalent Bonds
Exercise \(\PageIndex{1}\)
Which of the following groups of molecules contains no ionic compounds?
- HCN, NO2, and Ca(NO3)2
- PCl5, LiBr, and Zn(OH)2
- KOH, CCl4, and SF4
- NaH, CaF2, and NaNH2
- CH2O, H2S, and NH3
- Answer
-
e. CH2O, H2S, and NH3
Exercise \(\PageIndex{2}\)
Which combination of atoms is most likely to produce a compound with ionic bonds?
- B and C
- S and O
- N and F
- Si and Cl
- Mg and O
- Answer
-
e. Mg and O
Exercise \(\PageIndex{3}\)
Which combination of atoms is most likely to produce a compound with covalent bonds?
- K and Br
- Al and S
- S and Cl
- Sn and F
- Li and I
- Answer
-
c. S and Cl
Exercise \(\PageIndex{4}\)
Which of the following pairs of compounds contains ionic bonds?
- KI and O3
- NaF and H2O
- PCl5 and HF
- Na2SO3 and BH3
- RbBr and MgS
- Answer
-
e. RbBr and MgS
Exercise \(\PageIndex{5}\)
Which of the following statements is/are CORRECT?
- Ionic bonds form when one or more valence electrons are transferred from one atom to another.
- Covalent bonds involve sharing of electrons between atoms.
- In most covalently bonded compounds, electrons are NOT shared equally between the atoms.
a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 1, 2, and 3
- Answer
-
e. 1, 2, and 3
Covalent Bonding and Lewis Structures
Exercise \(\PageIndex{1}\)
Which of the following statements is/are CORRECT?
- Chemical reactions result in the gain, loss, or rearrangement of valence electrons.
- For main group elements, the number of valence electrons equals eight minus the element’s group number.
- Core electrons are not involved in bonding or in chemical reactions.
a. 1 only b. 2 only c. 3 only d. 1 and 3 e. 1, 2, and 3
- Answer
-
d. 1 and 3
Exercise \(\PageIndex{2}\)
The given representation of an atom is called the _____.
- a Lewis dot structure.
- an ion.
- a structural formula.
- an electrostatic potential map.
- an ionic bond.
- Answer
-
a. a Lewis dot structure.
Exercise \(\PageIndex{3}\)
In the Lewis formula for sulfur dioxide, SO2, the number of lone pairs of electrons around the sulfur atom is
- Answer
-
1.
Exercise \(\PageIndex{4}\)
What is the total number of electrons (both lone and bond pairs) in the Lewis structure of SO42-?
- Answer
-
32
Exercise \(\PageIndex{5}\)
How many valence electrons are present in the Lewis formula for the chlorite ion, Cl2–?
- Answer
-
20
Exercise \(\PageIndex{6}\)
Which of the following molecules or ions are isoelectronic: SO3, NF3, NO3–, CO32–?
- Answer
-
SO3, NO3– and CO32–
Exercise \(\PageIndex{7}\)
Which of the following has a Lewis structure similar to OCS?
a. NH2− b. O3 c. N2O+ d. SO2 e. ClO2−
- Answer
-
c. N2O+
Exercise \(\PageIndex{8}\)
The correct Lewis structure for Cl2CO is (the 2 Cl's and the O are bound to the C and not to each other):
a. 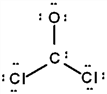 b.
b. 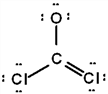 c.
c. 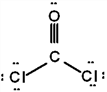 d.
d. 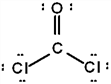 e. None of these
e. None of these
- Answer
-
a.

Exercise \(\PageIndex{9}\)
Which of the following is the Lewis electron dot structure for carbon monoxide?
a.  b.
b. 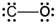 c.
c. 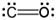 d.
d.  e.
e. 
- Answer
-
a.

Exercise \(\PageIndex{10}\)
How many hydrogen atoms are needed to complete the following hydrocarbon structure?
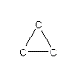
- Answer
-
12
Resonance Structures
Exercise \(\PageIndex{1}\)
In benzene, C6H6, the six carbon atoms are arranged in a ring. Two equivalent Lewis structures can be drawn for benzene. In both structures, the carbon atoms have a trigonal planar geometry. These two equivalent structures are referred to as ________ structures.
- Answer
-
resonance
Exercise \(\PageIndex{2}\)
Which of the following is not a valid resonance structure for N3–?
a. 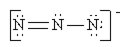 b.
b. 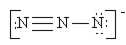 c.
c. 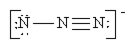 d.
d. 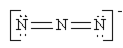 e. all are correct
e. all are correct
- Answer
-
a.
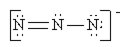
Exercise \(\PageIndex{3}\)
Which of the following are the correct resonance structures for the formate ion, HCO2–?
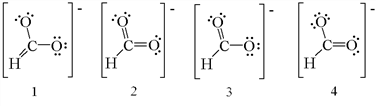
a. 1 and 2 b. 2 and 3 c. 3 and 4 d. 1, 3, and 4 e. 2, 3, and 4
- Answer
-
c. 3 and 4
Exercise \(\PageIndex{4}\)
Which of the following exhibits resonance?
a. SCl4 b. H2O c. SO2 d. BH3 e. none
- Answer
-
c. SO2
Exercise \(\PageIndex{5}\)
Which of the following molecules or ions does not have one or more resonance structures?
a. O3 b. OCN– c. SO2 d. H2CO e. NO3–
- Answer
-
d. H2CO
Exercise \(\PageIndex{1}\)
Which of the following statements concerning the formal charge of an atom is/are true?
- The formal charge of each individual atom in a molecule or ion is the actual atomic charge that can be determined experimentally.
- The formal charge of each individual atom is always the same for each possible resonance form.
- The sum of the formal charges of each atom in an ion equals the overall charge of the molecule or ion.
a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 1 and 3
- Answer
-
c. 3 only
Exercise \(\PageIndex{2}\)
What is the formal charge on the carbon atom in CO?
- Answer
-
0
Exercise \(\PageIndex{3}\)
What is the formal charge on each atom in dichloromethane, CH2Cl2?
- Answer
-
C atom = 0, each H atom = 0, and each Cl atom = 0
Exercise \(\PageIndex{4}\)
What is the formal charge on the O2 atom in the following Lewis structure?
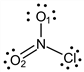
- Answer
-
b1
Exercise \(\PageIndex{5}\)
What is the formal charge of the oxygen atom in the Lewis structure for cyanate shown below?
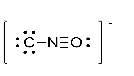
- Answer
-
2
Exercise \(\PageIndex{6}\)
One resonance structure for OCN– ion is drawn below. What is the formal charge on each atom?
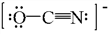
- Answer
-
O atom = –1, C atom = 0, and N atom = 0
Lewis Dot Structures, Electron and Molecular Geometry
Exercise \(\PageIndex{1}\)
Draw the best Lewis Dot Structure for each of the following species. Give the name of the electronic arrangement and the name for the molecular geometry for each of the species.
BeF2, BCl3, CCl4, PBr5, SI6, BH2–, NI3, ClF4+, SF5–
- Answer
-
Species Name Lewis Dot Structure Electron Geometry Molecular Geometry BeF2 linear linear BCl3 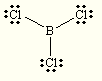
trigonal planar trigonal planar CCl4 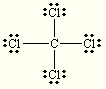
tetrahedral tetrahedral PBr5 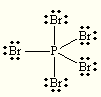
trigonal bipyramidal trigonal bipyramidal SI6 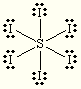
octahedral octahedral BH2– 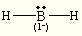
trigonal planar bent NI3 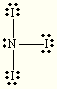
tetrahedral trigonal pyramidal ClF4+ trigonal bipyramidal see saw SF5– octahedral square pyramidal
Molecular Geometry
Exercise \(\PageIndex{1}\)
What is the molecular geometry around an atom in a molecule or ion which is surrounded by zero lone pairs of electrons and four single bonds.
- Answer
-
tetrahedral
Exercise \(\PageIndex{2}\)
What is the electron-pair geometry around an atom in a molecule or ion which is surrounded by two lone pairs of electrons and three single bonds.
- Answer
-
trigonal bipyramidal
Exercise \(\PageIndex{3}\)
The molecular geometry of a molecule whose central atom has four single bonds and one lone pair of electrons is ________.
- Answer
-
trigonal-bipyramidal
Exercise \(\PageIndex{4}\)
What is the bond angle in a trigonal planar molecule or ion?
- Answer
-
120°
VSEPR
Exercise \(\PageIndex{5}\)
What is the molecular geometry around the nitrogen atom as per the valence shell electron-pair repulsion (VSEPR) theory??
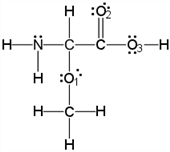
- Answer
-
trigonal pyramidal
Exercise \(\PageIndex{6}\)
What is the molecular geometry around carbon atom C1?
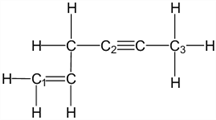
- Answer
-
trigonal planar
Exercise \(\PageIndex{7}\)
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of nitrogen trichloride, NCl3.
- The electron-pair geometry is linear, the molecular geometry is linear.
- The electron-pair geometry is trigonal-planar, the molecular geometry is trigonal-planar.
- The electron-pair geometry is trigonal-planar, the molecular geometry is bent.
- The electron-pair geometry is tetrahedral, the molecular geometry is tetrahedral.
- The electron-pair geometry is tetrahedral, the molecular geometry is trigonal-pyramidal.
- Answer
-
e. The electron-pair geometry is tetrahedral, the molecular geometry is trigonal-pyramidal.
Exercise \(\PageIndex{8}\)
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of boron tribromide, BBr3.
- The electron-pair geometry is trigonal-pyramidal, the molecular geometry is trigonal-pyramidal.
- The electron-pair geometry is trigonal-planar, the molecular geometry is trigonal-planar.
- The electron-pair geometry is trigonal-planar, the molecular geometry is bent.
- The electron-pair geometry is tetrahedral, the molecular geometry is trigonal-pyramidal.
- The electron-pair geometry is trigonal-pyramidal, the molecular geometry is t-shaped.
- Answer
-
b. The electron-pair geometry is trigonal-planar, the molecular geometry is trigonal-planar.
Bond Angles
Exercise \(\PageIndex{9}\)
Based on electron-pair geometries, which of the following molecules has the smallest bond angle between any two adjacent atoms?
a. CH4 b. H2O c. BH3 d. PH3 e. SF6
- Answer
-
e. SF6
Exercise \(\PageIndex{10}\)
What are the approximate H−N−H bond angles in NH4+?
- Answer
-
109.5°
Exercise \(\PageIndex{11}\)
What is the O−C−N bond angle in OCN–?
- Answer
-
180°
Exercise \(\PageIndex{12}\)
What are the approximate F−Br−F bond angles in BrF5?
- Answer
-
90° and 180°
Exercise \(\PageIndex{13}\)
In PCl5, the Cl−P−Cl bond angle between an axial and an equatorial chlorine atom is ________ degrees.
- Answer
-
90
Exercise \(\PageIndex{14}\)
Place the following molecules in order from smallest to largest H−N−H bond angles: NH4+, NH3, and NH2–.
- NH4+ < NH3 < NH2–
- NH4+ < NH2– < NH3
- NH2– < NH3 < NH4+
- NH2– < NH4+ < NH3
- NH3 < NH2– < NH4+
- Answer
-
c. NH2– < NH3 < NH4+
Bond Polarity and Electronegativity
Exercise \(\PageIndex{1}\)
Which of the following bonds would be the most polar?
a. N--C b. N--Si c. N--P d. N--Al e. N--Ga
- Answer
-
e. N--Ga
Exercise \(\PageIndex{2}\)
Which of the following compounds has polar covalent bonds: CCl4, Cl2, HCl, and KCl?
- Answer
-
CCl4 and HCl
Exercise \(\PageIndex{3}\)
Electronegativity is a measure of _____.
- the ability of a substance to conduct electricity
- the charge on a polyatomic cation
- the charge on a polyatomic anion
- the ability of an atom in a molecule to attract electrons to itself
- the oxidation number of an atom in a molecule or polyatomic anion
- Answer
-
d. the ability of an atom in a molecule to attract electrons to itself
Exercise \(\PageIndex{4}\)
Three nonequivalent Lewis structures for carbonyl sulfide, SCO, are given below. Use the concepts of formal charge and electronegativity to choose the structure that is the best representation.
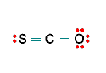
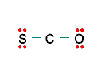
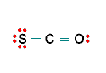
A B C
- Structure A, because all the formal charges equal 0
- Structure B, because all the formal charges equal 0
- Structure C, because all the formal charges equal 0.
- Structure A, because the negative formal charge resides on the most electronegative atom
- Structure C, because the negative formal charge resides on the most electronegative atom
- Answer
-
b. Structure B, because all the formal charges equal 0
Exercise \(\PageIndex{5}\)
Choose which central atom in the following molecules is most electronegative.
a. PH3 b. CH4 c. H2S d. H2O e. NH3
- Answer
-
d. H2O
Exercise \(\PageIndex{6}\)
Rank the following covalent bonds in order of decreasing polarity: C-H, N-H, O-H, and F-H.
- Answer
-
F-H > O-H > N-H > C-H
Exercise \(\PageIndex{7}\)
Atoms having equal or nearly equal electronegativities are expected to form
- no bonds
- polar covalent bonds
- nonpolar covalent bonds
- ionic bonds
- covalent bonds
- Answer
-
c. nonpolar covalent bonds
Bond and Molecular Polarity
Exercise \(\PageIndex{1}\)
If a molecule has a positive and negative end, the molecule is said to have a(n) ________ moment.
- Answer
-
dipole
Exercise \(\PageIndex{1}\)
Which of the following molecules is nonpolar?
- sulfur dioxide, SO2
- hydrogen fluoride, HF
- phosphorus trifluoride, PF3
- boron trifluoride, BF3
- iodine trichloride, ICl3
- Answer
-
d. boron trifluoride, BF3
Exercise \(\PageIndex{2}\)
Which of the following molecules is polar?
a. CS2 b. SO2 c. XeF2 d. XeF4 e. SO3
- Answer
-
b. SO2
Exercise \(\PageIndex{3}\)
Three possible structures of C2H2Cl2 are shown below. Which of these molecules are polar?
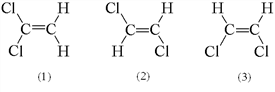
a. 1 only b. 2 only c. 3 only d. 1 and 3 e. 2 and 3
- Answer
-
d. 1 and 3
Bond Order
Exercise \(\PageIndex{1}\)
Linus Pauling noticed that the energy of a polar bond is often greater than expected. He attributed the greater bond energy to
- a coulombic attraction between atoms with partially positive and negative charges.
- the greater bond lengths of the heteronuclear bonds.
- one of the many unexplainable phenomena that scientists encounter.
- the ability of heteronuclear species to form double and triple bonds.
- the greater number of valence electrons found in heteronuclear molecules.
- Answer
-
a. a coulombic attraction between atoms with partially positive and negative charges.
Exercise \(\PageIndex{2}\)
In molecules, as bond order increases,
- both bond length and bond energy increase.
- both bond length and bond energy decrease.
- bond length increases and bond energy is unchanged.
- bond length is unchanged and bond energy increases.
- bond length decreases and bond energy increases.
- Answer
-
e. bond length decreases and bond energy increases.
Exercise \(\PageIndex{3}\)
Determine the bond order of NO+?
a. 2 b. 1.5 c. 1 d. 2.5 e. 3
- Answer
-
e. 3
Exercise \(\PageIndex{4}\)
Use Lewis structures to predict the bond order for a sulfur-oxygen bond in SO32-?
a. 1 b. 4/3 c. 5/2 d. 2 e. 3/2
- Answer
-
a. 1
Exercise \(\PageIndex{5}\)
Use Lewis structures to predict the bond order for a sulfur-oxygen bond in the sulfite ion?
a. 1 b. 3/2 c. 4/3 d. 2 e. 5/2
- Answer
-
c. 4/3
Bond Energy
Exercise \(\PageIndex{6}\)
Based on the following data, what is the F-F bond energy?
H2(g) + F2(g) → HF(g); ΔrH = –272.5 kJ/mol-rxn
Bond Bond Energy (kJ/mol)
H-H 435
H-F 565
a. 500 kJ/mol b. 150 kJ/mol c. –150 kJ/mol d. –695 kJ/mol e. 695 kJ/mol
- Answer
-
b. 150 kJ/mol
Cleavage of one H-H bond, \(\Delta H_{1}= 435 kJ/mol;\)
Cleavage of one H-F bond, \(\Delta H_{2}= 565 kJ/mol;\)
Formation of two F-F bonds, \(\Delta H_{3}= ?\)
ΔHf of the reaction is = -272.5 kJ/mol = -545 kJ/2 mol
\(\Delta H_{f}=(\Delta H_{1}+\Delta H_{3})-(2*\Delta H_{2})\)
\(-545 = (435+\Delta H_{3})-(2*565)\)
\(-545 = (435+\Delta H_{3})-1130\)
\(585 = 435+\Delta H_{3}\)\(153 kJ = \Delta H_{3}\)
Exercise \(\PageIndex{7}\)
Using bond-energy data, what is ΔrH for the following reaction?
CH4(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
Bond Bond Energy (kJ/mol)
C-H 413
H-H 432
Cl-Cl 242
C-Cl 328
a. –40 kJ/mol-rxn b. –150 kJ/mol-rxn c. 40 kJ/mol-rxn d. 1415 kJ/mol-rxn e. 150 kJ/mol-rxn
- Answer
-
a. –40 kJ/mol-rxn
Exercise \(\PageIndex{8}\)
Using the following data reactions:
ΔH° (kJ/mol-rxn)
H2(g) + Cl2(g) → 2HCl(g) –184
H2(g) → 2H(g) 432
Cl2(g) → 2Cl(g) 239
calculate the energy of an H-Cl bond.
a. 92 kJ/mol b. 855 kJ/mol c. 487 kJ/mol d. 428 kJ/mol e. 244 kJ/mol
- Answer
-
d. 428 kJ/mol
Exercise \(\PageIndex{9}\)
Which of the following species has the shortest carbon−nitrogen bond?
a. CH3CN b. CH3N2 c. CH2NH d. CH3CONH2 e. CH3NH3+
- Answer
-
a. CH3CN
Exercise \(\PageIndex{10}\)
Use the bond energies provided to complete the following statement.
________ when all of the bonds in acetic acid (CH3COOH) are broken.
Bond Bond Energy (kJ/mol)
C-H 413
C-O 358
O-H 463
C=O 745
C-C 348
C=C 614
- 3153 kJ/mol of energy is consumed
- 3153 kJ/mol of energy is released
- 2805 kJ/mol of energy is released
- 2805 kJ/mol of energy is consumed
- 2766 kJ/mol of energy is consumed
- Answer
-
a. 3153 kJ/mol of energy is consumed
DNA, Revisited
Exercise \(\PageIndex{1}\)
The backbone of DNA consists of the atoms O—P—O—C—C—C. Each atom has a(n) _____ electron-pair geometry.
- tetrahedral
- trigonal bipyramidal
- trigonal-planar
- linear
- octahedra
- Answer
-
a. tetrahedral
Exercise \(\PageIndex{2}\)
The rings in the nitrogen-containing bases in DNA are all flat with _____ electron-pair geometry around each atom.
- trigonal-planar
- tetrahedral
- trigonal bipyramidal
- linear
- octahedral
- Answer
-
a. trigonal-planar