9.1: Glycolysis - Reaction and Regulation
- Page ID
- 166215
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Glycolysis is the first step in the breakdown of glucose to extract energy for cellular metabolism. Nearly all living organisms carry out glycolysis as part of their metabolism. The process does not use oxygen and is therefore anaerobic. Glycolysis takes place in the cytoplasm of both prokaryotic and eukaryotic cells. Glucose enters heterotrophic cells in two ways. One method is through secondary active transport in which the transport takes place against the glucose concentration gradient. The other mechanism uses a group of integral proteins called GLUT proteins, also known as glucose transporter proteins. These transporters assist in the facilitated diffusion of glucose.
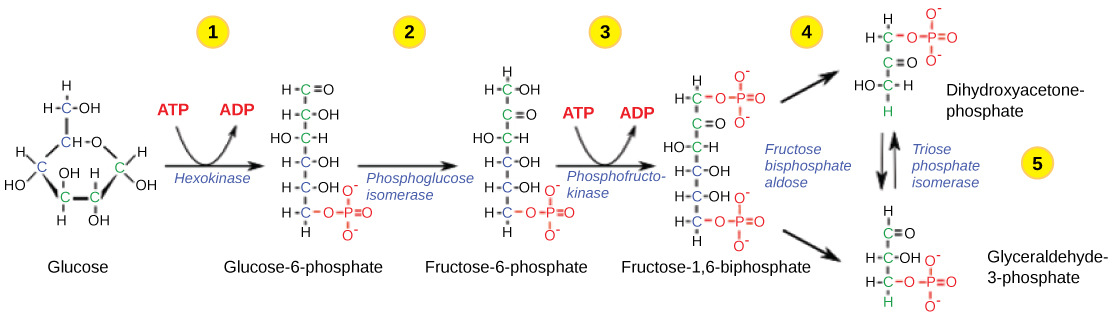
First Half of Glycolysis (Energy-Requiring Steps)
Step 1. The first step in glycolysis (Figure 9.1.1) is catalyzed by hexokinase, an enzyme with broad specificity that catalyzes the phosphorylation of six-carbon sugars. Hexokinase phosphorylates glucose using ATP as the source of the phosphate, producing glucose-6-phosphate, a more reactive form of glucose. This reaction prevents the phosphorylated glucose molecule from continuing to interact with the GLUT proteins, and it can no longer leave the cell because the negatively charged phosphate will not allow it to cross the hydrophobic interior of the plasma membrane.
Step 2. In the second step of glycolysis, an isomerase converts glucose-6-phosphate into one of its isomers, fructose-6-phosphate. An isomerase is an enzyme that catalyzes the conversion of a molecule into one of its isomers. (This change from phosphoglucose to phosphofructose allows the eventual split of the sugar into two three-carbon molecules.).
Step 3. The third step is the phosphorylation of fructose-6-phosphate, catalyzed by the enzyme phosphofructokinase. A second ATP molecule donates a high-energy phosphate to fructose-6-phosphate, producing fructose-1,6-bisphosphate. In this pathway, phosphofructokinase is a rate-limiting enzyme. It is active when the concentration of ADP is high; it is less active when ADP levels are low and the concentration of ATP is high. Thus, if there is “sufficient” ATP in the system, the pathway slows down. This is a type of end product inhibition, since ATP is the end product of glucose catabolism.
Step 4. The newly added high-energy phosphates further destabilize fructose-1,6-bisphosphate. The fourth step in glycolysis employs an enzyme, aldolase, to cleave 1,6-bisphosphate into two three-carbon isomers: dihydroxyacetone-phosphate and glyceraldehyde-3-phosphate.
Step 5. In the fifth step, an isomerase transforms the dihydroxyacetone-phosphate into its isomer, glyceraldehyde-3-phosphate. Thus, the pathway will continue with two molecules of a single isomer. At this point in the pathway, there is a net investment of energy from two ATP molecules in the breakdown of one glucose molecule.
Second Half of Glycolysis (Energy-Releasing Steps)
So far, glycolysis has cost the cell two ATP molecules and produced two small, three-carbon sugar molecules. Both of these molecules will proceed through the second half of the pathway, and sufficient energy will be extracted to pay back the two ATP molecules used as an initial investment and produce a profit for the cell of two additional ATP molecules and two even higher-energy NADH molecules.
Step 6. The sixth step in glycolysis (Figure 9.1.2) oxidizes the sugar (glyceraldehyde-3-phosphate), extracting high-energy electrons, which are picked up by the electron carrier NAD+, producing NADH. The sugar is then phosphorylated by the addition of a second phosphate group, producing 1,3-bisphosphoglycerate. Note that the second phosphate group does not require another ATP molecule.
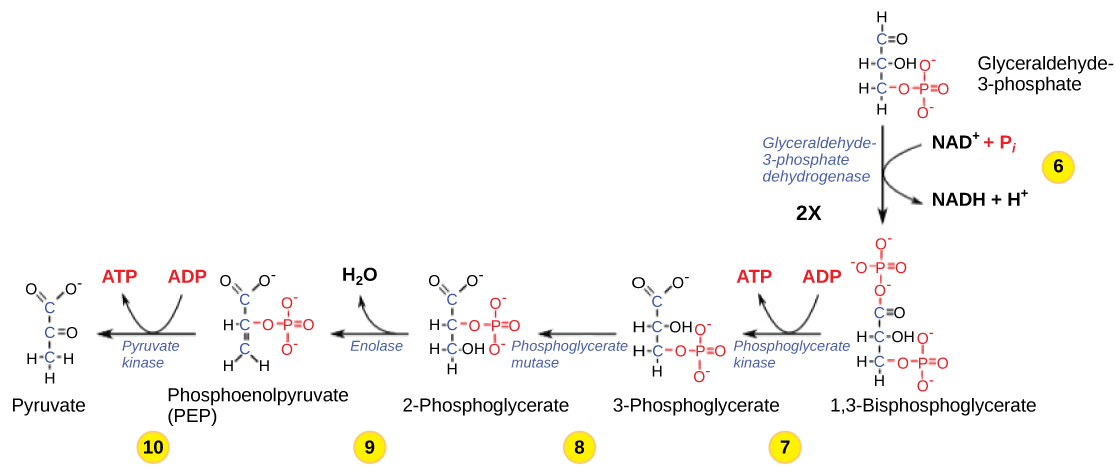
Here again is a potential limiting factor for this pathway. The continuation of the reaction depends upon the availability of the oxidized form of the electron carrier, NAD+. Thus, NADH must be continuously oxidized back into NAD+ in order to keep this step going. If NAD+ is not available, the second half of glycolysis slows down or stops. If oxygen is available in the system, the NADH will be oxidized readily, though indirectly, and the high-energy electrons from the hydrogen released in this process will be used to produce ATP. In an environment without oxygen, an alternate pathway (fermentation) can provide the oxidation of NADH to NAD+.
Step 7. In the seventh step, catalyzed by phosphoglycerate kinase (an enzyme named for the reverse reaction), 1,3-bisphosphoglycerate donates a high-energy phosphate to ADP, forming one molecule of ATP. (This is an example of substrate-level phosphorylation.) A carbonyl group on the 1,3-bisphosphoglycerate is oxidized to a carboxyl group, and 3-phosphoglycerate is formed.
Step 8. In the eighth step, the remaining phosphate group in 3-phosphoglycerate moves from the third carbon to the second carbon, producing 2-phosphoglycerate (an isomer of 3-phosphoglycerate). The enzyme catalyzing this step is a mutase (isomerase).
Step 9. Enolase catalyzes the ninth step. This enzyme causes 2-phosphoglycerate to lose water from its structure; this is a dehydration reaction, resulting in the formation of a double bond that increases the potential energy in the remaining phosphate bond and produces phosphoenolpyruvate (PEP).
Step 10. The last step in glycolysis is catalyzed by the enzyme pyruvate kinase (the enzyme in this case is named for the reverse reaction of pyruvate’s conversion into PEP) and results in the production of a second ATP molecule by substrate-level phosphorylation and the compound pyruvic acid (or its salt form, pyruvate). Many enzymes in enzymatic pathways are named for the reverse reactions, since the enzyme can catalyze both forward and reverse reactions (these may have been described initially by the reverse reaction that takes place in vitro, under non-physiological conditions).
The net reaction in the transformation of glucose into pyruvate is:

Thus, two molecules of ATP are generated in the conversion of glucose into two molecules of pyruvate.
Note that the energy released in the anaerobic conversion of glucose into two molecules of pyruvate is -21 kcal mol-1 (- 88 kJ mol-1).
The Fates of Pyruvate
Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or to fatty acids through acetyl-CoA. It can also be used to construct the amino acid alanine, and it can be converted into ethanol.
Pyruvic acid supplies energy to living cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration); when oxygen is lacking, it ferments to produce lactic acid. Pyruvate is an important chemical compound in biochemistry. It is the output of the anaerobic metabolism of glucose known as glycolysis. One molecule of glucose breaks down into two molecules of pyruvate, which are then used to provide further energy in one of two ways. Pyruvate is converted into acetyl- coenzyme A, which is the main input for a series of reactions known as the Krebs cycle.
The net reaction of converting pyruvate into acetyl CoA and CO2 is:

Pyruvate is also converted to oxaloacetate by an anaplerotic reaction, which replenishes Krebs cycle intermediates; also, oxaloacetate is used for gluconeogenesis. These reactions are named after Hans Adolf Krebs, the biochemist awarded the 1953 Nobel Prize for physiology, jointly with Fritz Lipmann, for research into metabolic processes. The cycle is also known as the citric acid cycle or tri-carboxylic acid cycle, because citric acid is one of the intermediate compounds formed during the reactions.
If insufficient oxygen is available, the acid is broken down anaerobically, creating lactate in animals and ethanol in plants and microorganisms. Pyruvate from glycolysis is converted by fermentation to lactate using the enzyme lactate dehydrogenase and the coenzyme NADH in lactate fermentation. Alternatively it is converted to acetaldehyde and then to ethanol in alcoholic fermentation.
Pyruvate is a key intersection in the network of metabolic pathways. Pyruvate can be converted into carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine, and to ethanol. Therefore, it unites several key metabolic processes.
Regulation

Figure 9.1.3: Glycolysis Regulation
Control of glycolysis is unusual for a metabolic pathway, in that regulation occurs at three enzymatic points:
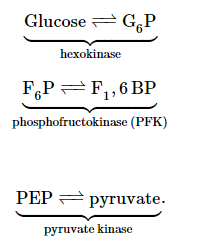
Glycolysis is regulated in a reciprocal fashion compared to its corresponding anabolic pathway, gluconeogenesis. Reciprocal regulation occurs when the same molecule or treatment (phosphorylation, for example) has opposite effects on catabolic and anabolic pathways. Reciprocal regulation is important when anabolic and corresponding catabolic pathways are occurring in the same cellular location.
As an example, consider regulation of PFK. It is activated by several molecules, most importantly fructose-2,6- bisphosphate (F2,6BP). This molecule has an inhibitory effect on the corresponding gluconeogenesis enzyme, fructose-1,6-bisphosphatase (F1,6BPase).
You might wonder why pyruvate kinase, the last enzyme in the pathway, is regulated. The answer is simple. Pyruvate kinase catalyzes the most energetically rich reaction of glycolysis. The reaction is favored so strongly in the forward direction that cells must do a ‘two-step’ around it in the reverse direction when making glucose. In other words, it takes two enzymes, two reactions, and two triphosphates to go from pyruvate back to PEP in gluconeogenesis. When cells are needing to make glucose, they can’t be sidetracked by having the PEP they have made in gluconeogenesis be converted directly back to pyruvate by pyruvate kinase. Consequently, pyruvate kinase is inhibited during gluconeogenesis, lest a “futile cycle" occur.
Another interesting control mechanism called feedforward activation involves pyruvate kinase. Pyruvate kinase is activated allosterically by F1,6BP. This molecule is a product of the PFK reaction and a substrate for the aldolase reaction. It should be noted that the aldolase reaction is energetically unfavorable (high +ΔΔG°’), thus allowing F1,6BP to accumulate. When this happens, some of the excess F1,6BP activates pyruvate kinase, which jump-starts the conversion of PEP to pyruvate. The resulting drop in PEP levels has the effect of “pulling" on the reactions preceding pyruvate kinase. As a consequence, the concentrations of G3P and DHAP fall, helping to move the aldolase reaction forward.
Outcomes of Glycolysis
Glycolysis starts with one molecule of glucose and ends with two pyruvate (pyruvic acid) molecules, a total of four ATP molecules, and two molecules of NADH. Two ATP molecules were used in the first half of the pathway to prepare the six-carbon ring for cleavage, so the cell has a net gain of two ATP molecules and 2 NADH molecules for its use. If the cell cannot catabolize the pyruvate molecules further (via the citric acid cycle or Krebs cycle), it will harvest only two ATP molecules from one molecule of glucose.
Mature mammalian red blood cells do not have mitochondria and are not capable of aerobic respiration, the process in which organisms convert energy in the presence of oxygen. Instead, glycolysis is their sole source of ATP. Therefore, if glycolysis is interrupted, the red blood cells lose their ability to maintain their sodium-potassium pumps, which require ATP to function, and eventually, they die. For example, since the second half of glycolysis (which produces the energy molecules) slows or stops in the absence of NAD+, when NAD+ is unavailable, red blood cells will be unable to produce a sufficient amount of ATP in order to survive.
Additionally, the last step in glycolysis will not occur if pyruvate kinase, the enzyme that catalyzes the formation of pyruvate, is not available in sufficient quantities. In this situation, the entire glycolysis pathway will continue to proceed, but only two ATP molecules will be made in the second half (instead of the usual four ATP molecules). Thus, pyruvate kinase is a rate-limiting enzyme for glycolysis.
Contributors
- Darik Benson, (University California Davis)
- Dr. Kevin Ahern and Dr. Indira Rajagopal (Oregon State University)

