8.2: Monosaccharides
- Page ID
- 165307
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A monosaccharide is a carbohydrate consisting of one sugar unit. Common examples of simple sugars or monosaccharides are glucose and fructose. Both of these monosaccharides are referred to as hexoses since they have six carbons. Glucose is abundant in many plant sources and makes up sweeteners such as corn sugar or grape sugar. Fructose found in honey and fruits. These sugars are structural isomers of one another, with the difference being that glucose contains an aldehyde functional group whereas fructose contains a ketone functional group.
Figure 8.2.1:Glucose and fructose are monosaccharides, or simple sugars.
Glucose and fructose are both soluble in water. In aqueous solution, the predominant forms are not the straight-chain structure shown above. Rather, they adopt a cyclic structure (see figure below). Glucose is six membered ring, while fructose is a five-membered ring. Both rings contain an oxygen atom.
Figure 8.2.1: The cyclic form of sugars is the favored form in aqueous solution.
Glucose and other 5C and 6C sugars can cyclize through intramolecular nucleophilic attack of one of the OH's on the carbonyl C of the aldehyde or ketone. Such intramolecular reactions occur if stable 5 or 6 member rings can form. The resulting rings are labeled furanose (5 member) or pyranose (6 member) based on their similarity to furan and pyran. On nucleophilic attack to form the ring, the carbonyl O becomes an OH which points either below the ring (a anomer) or above the ring (b anomer).
Monosaccharides in solution exist as equilibrium mixtures of the straight and cyclic forms. In solution, glucose is mostly in the pyranose form, fructose is 67% pyranose and 33% furanose, and ribose is 75% furanose and 25% pyranose.
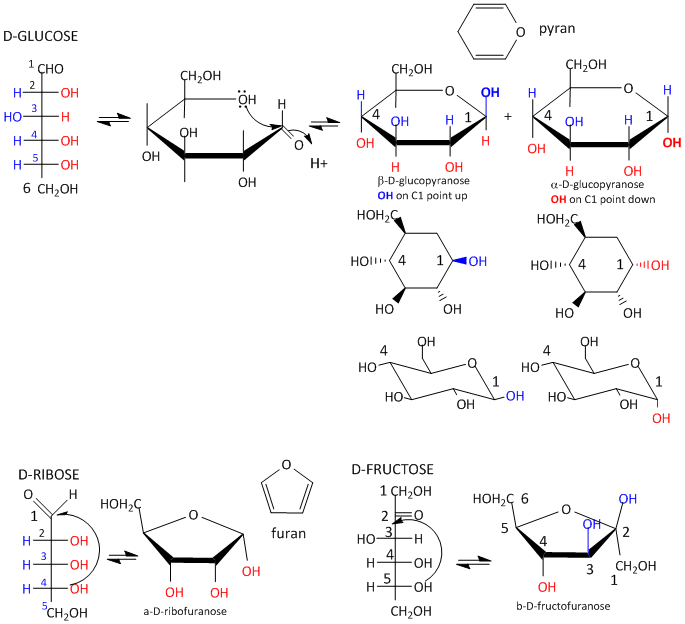
Sugars can be drawn in the straight chain form as either Fisher projections or perspective structural formulas.
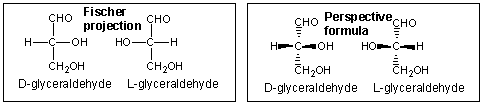
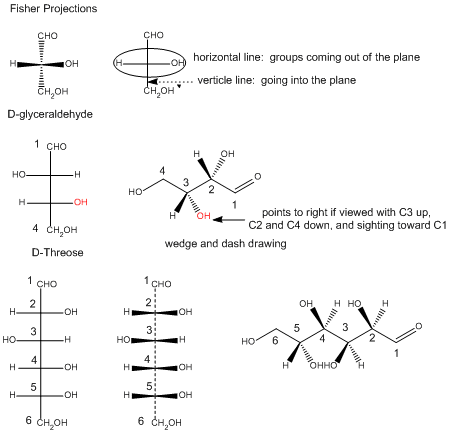
In the Fisher projection, the vertical bonds point down into the plane of the paper. That's easy to visualize for 3C molecules. but more complicated for bigger molecules. For those draw a wedge and dash line drawing of the molecule. When determining the orientation of the OHs on each C, orient the wedge and dash drawing in your mind so that the C atoms adjacent to the one of interest are pointing down. Sighting towards the carbonyl C, if the OH is pointing to the right in the Fisher project, it should be pointing to the right in the wedge and dash drawing, as shown below for D-erthyrose and D-glucose.
Cyclic forms can be drawn either as the Haworth projections, which shows the molecule as cyclic and planar with substituents above or below the ring or the more plausible bent forms (showing Glucose in the chair or boat conformations, for example, b-D-glucopyranose is the only aldohexose which can be drawn with all its bulky substituents (OH and CH2OH) in equatorial positions, which probably accounts for its widespread prevalence in nature.
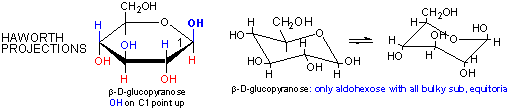
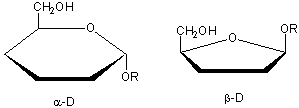
Haworth projections are more realistic than the Fisher projections, but you should be able to draw both structures. In general, if a substituent points to the right in the Fisher structure, it points down in the Haworth. if it points left, it points up. In general, the OH on the a-anomer points down (ants down) while on the b-anomer it points up (butterflies up).
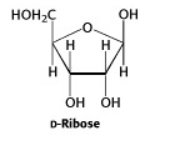
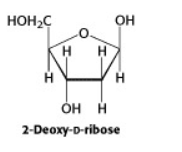
Another important group of monosaccharides are the pentoses, containing five carbons in the chain. Ribose and deoxyribose are two pentoses that are components of the structures of DNA and RNA.
Glycosides
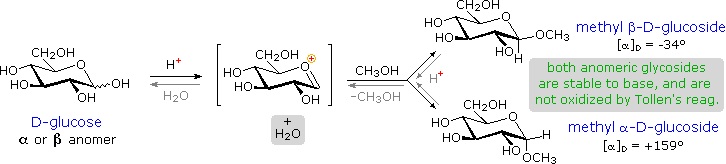
Acetal derivatives formed when a monosaccharide reacts with an alcohol in the presence of an acid catalyst are called glycosides. This reaction is illustrated for glucose and methanol in the diagram below. In naming of glycosides, the "ose" suffix of the sugar name is replaced by "oside", and the alcohol group name is placed first. As is generally true for most acetals, glycoside formation involves the loss of an equivalent of water. The diether product is stable to base and alkaline oxidants such as Tollen's reagent. Since acid-catalyzed aldolization is reversible, glycosides may be hydrolyzed back to their alcohol and sugar components by aqueous acid.
The anomeric methyl glucosides are formed in an equilibrium ratio of 66% alpha to 34% beta. From the structures in the previous diagram, we see that pyranose rings prefer chair conformations in which the largest number of substituents are equatorial. In the case of glucose, the substituents on the beta-anomer are all equatorial, whereas the C-1 substituent in the alpha-anomer changes to axial. Since substituents on cyclohexane rings prefer an equatorial location over axial (methoxycyclohexane is 75% equatorial), the preference for alpha-glycopyranoside formation is unexpected, and is referred to as the anomeric effect.
Glycosides abound in biological systems. By attaching a sugar moiety to a lipid or benzenoid structure, the solubility and other properties of the compound may be changed substantially. Because of the important modifying influence of such derivatization, numerous enzyme systems, known as glycosidases, have evolved for the attachment and removal of sugars from alcohols, phenols and amines. Chemists refer to the sugar component of natural glycosides as the glycon and the alcohol component as the aglycon.
Two examples of naturally occurring glycosides and one example of an amino derivative are displayed above. Salicin, one of the oldest herbal remedies known, was the model for the synthetic analgesic aspirin. A large class of hydroxylated, aromatic oxonium cations called anthocyanins provide the red, purple and blue colors of many flowers, fruits and some vegetables. Peonin is one example of this class of natural pigments, which exhibit a pronounced pH color dependence. The oxonium moiety is only stable in acidic environments, and the color changes or disappears when base is added. The complex changes that occur when wine is fermented and stored are in part associated with glycosides of anthocyanins. Finally, amino derivatives of ribose, such as cytidine play important roles in biological phosphorylating agents, coenzymes and information transport and storage materials.
Boat/Chair Conformations
Independent of stereoisomerization, sugars in ring form of a given type (such as glucose) can “twist" themselves into alternative conformations called boat and chair. Note that this rearrangement does not change the relative positions of hydroxyl groups. All that has changed is the shape of the molecule. As shown for glucose, one can see that the beta-hydroxyl of glucose is closer to the CH2OHCH2OH (carbon #6) in the boat form than it is in the chair form. Steric hindrance can be a factor in favoring one configuration over another.
Contributors
Dr. Kevin Ahern and Dr. Indira Rajagopal (Oregon State University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry



