2.6: Amino Acids and Proteins (Exercises)
- Page ID
- 165269
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Questions
1.1: Amino Acids
Q1.Read the material at:
https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/CHEM_4320%2F%2F5320%3A_Biochemistry_1/02%3A__Protein_Structure/2.6%3A_Amino_Acids_and_Proteins_(Exercises) and answer the following questions:
a. What are essential amino acids?
b. What are nonessential amino acids?
c. What happens if you are deficient in an amino acid?
Q2. Draw the functional groups present in all amino acids.
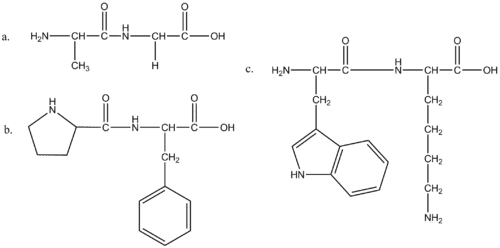
Q3. Complete the following for threonine, lysine, and tyrosine.
a. Draw the amino acid.
b. Circle the side chain.
c. Identify whether it is polar, nonpolar, acidic, or basic.
d. At what pH will it exist as a zwitterion?
e. What is the range of pH values when it will be positively charged?
f. What is the range of pH values when it will be negatively charged?
1.5: Peptides
Q4. Draw the two dipeptides formed from each pair of amino acids.
a. tyrosine and lysine
b. threonine and gluatmine
c. alanine and histidine
Q5. Draw and give the full names of the amino acids in the following dipeptides.
Q6. List of all of the possible polypeptides that can be formed from threonine, alanine, and phenylalanine (use three character abbreviations for each amino acid).
Q7. Draw the following polypeptides.
a. Ser-Tyr-Gln
b. Lys-Met-Gly
Q8. Identify each of the amino acids in the polypeptide and then name it using the three character abbreviations.
2.1: Protein Structure
Q9. Describe the four levels of protein structure.
Q10. What levels of structure involve hydrogen bonding?
Q11. What types of structure is the result of interactions between amino acids that are far apart in the primary structure?
Q12. What types of interactions hold the secondary structure together?
Q13. What types of interactions hold the tertiary structure together?
Q14. What levels of structure are affected by denaturation?
Q15. A protein has one subunit. Would it have a quaternary structure?
Answers
1.1: Amino Acids
Q1
a. Essential amino acids are those you get from your diet.
b. Nonessential amino acids are produced in the body.
c. Illness and/or degradation of body's proteins.
Q2
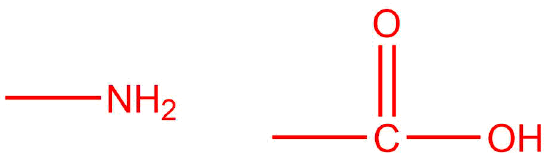
amine and carboxylic acid
Q3
Complete the following for threonine, lysine, and tyrosine.
threonine
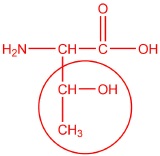
- polar
- 5.60
- < 5.60
- > 5.60
lysine
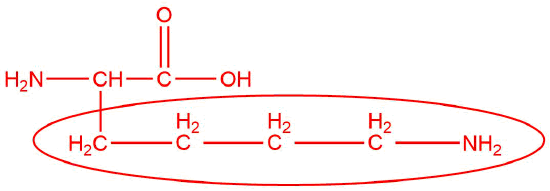
- basic
- 9.47
- < 9.47
- > 9.47
tyrosine
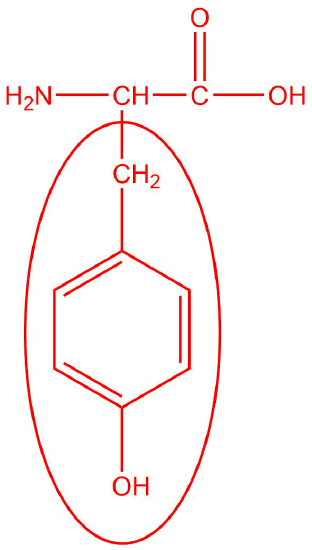
- polar
- 5.63
- < 5.63
- > 5.63
1.5: Peptides
Q4
Draw the two dipeptides formed from each pair of amino acids.
a. 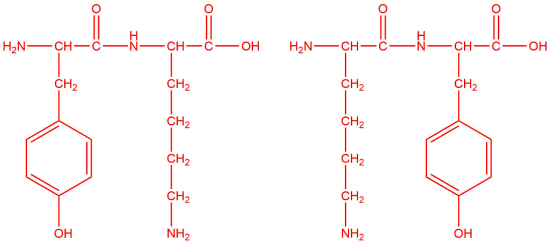
b. 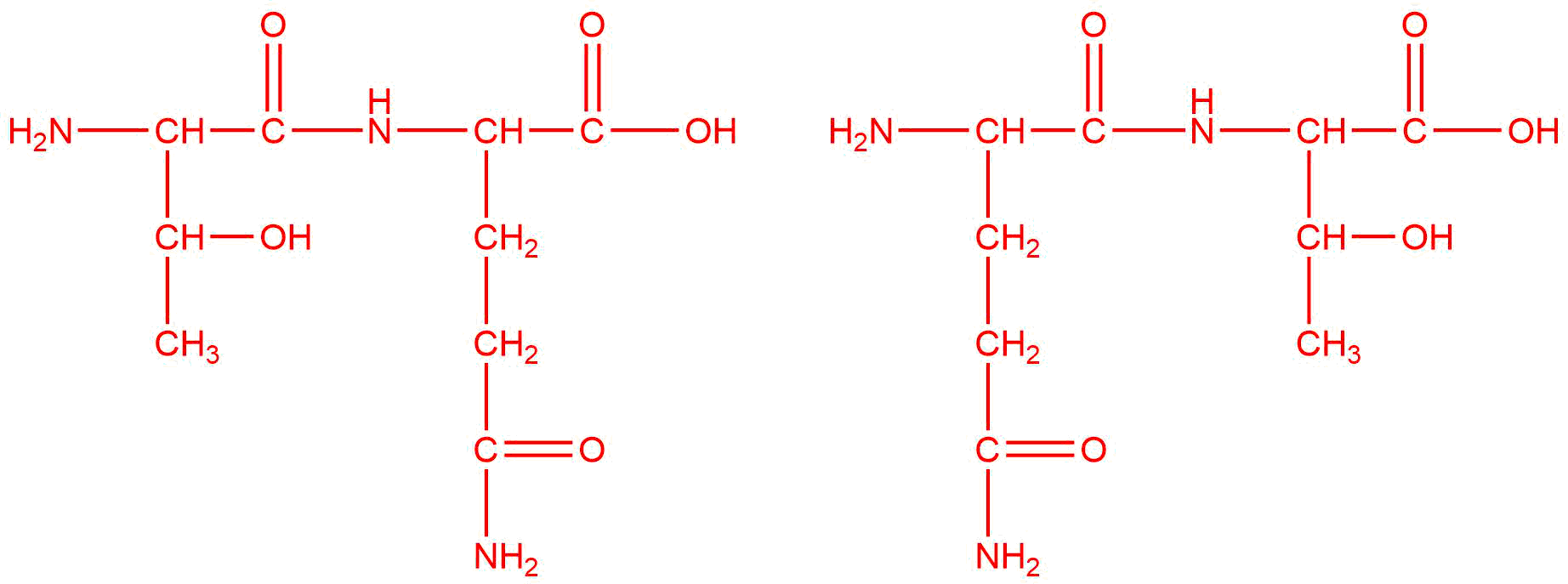
c. 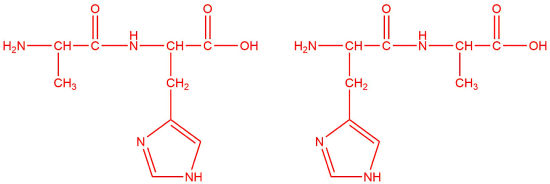
Q5
| a. | alanine 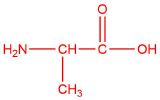 |
glycine 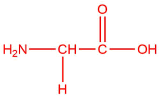 |
| b. | proline 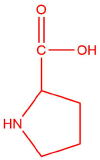 |
phenylalanine 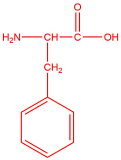 |
| c. | tryptophan 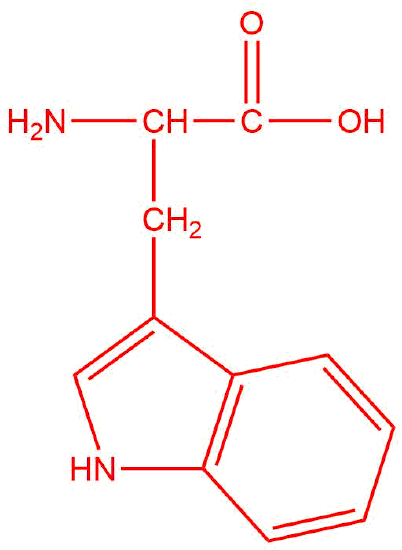 |
lysine 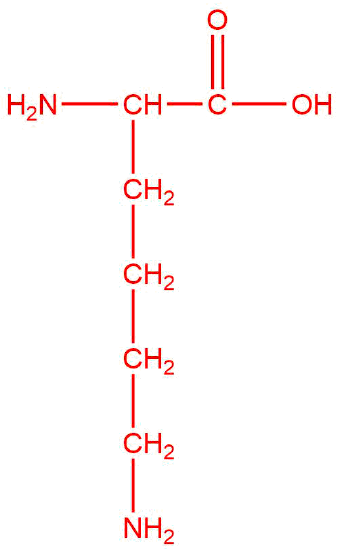 |
Q6
Thr-Ala-Phe
Thr-Phe-Ala
Ala-Thr-Phe
Ala-Phe-Thr
Phe-Ala-Thr
Phe-Thr-Ala
Q7
a. 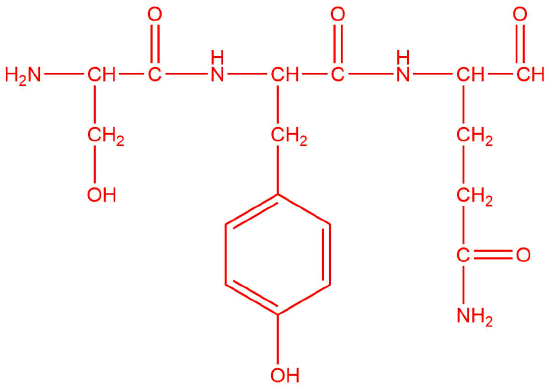
b. 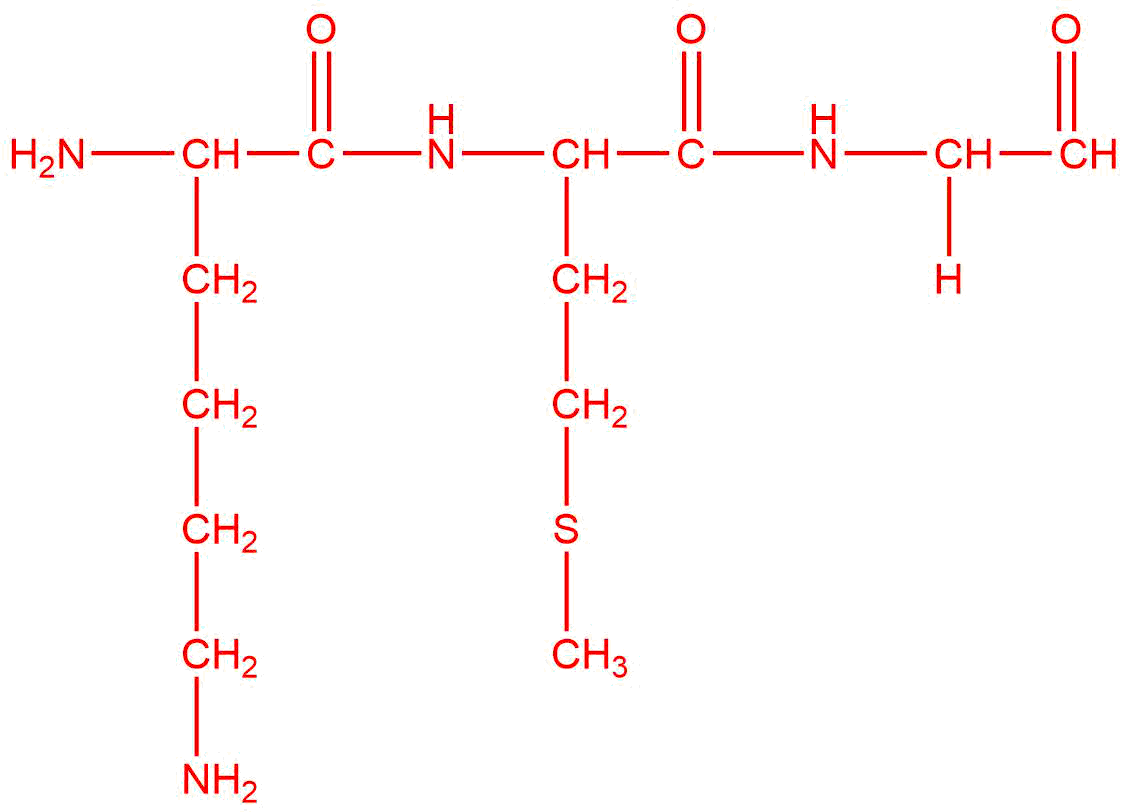
Q8
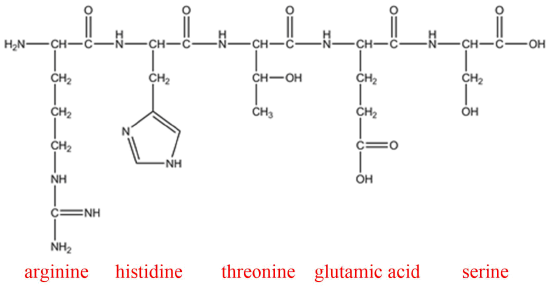
Arg-His-Thr-Glu-Ser
2.2: Protein Structure
Q9
Primary - sequence of amino acids
Secondary - alpha helix and Beta-pleated sheets held together by hydrogen bonds
Tertiary - third level of structure of protein often forming globular or fibrous structure, held together by variety of attractive forces
Quaternary - complex of multiple proteins held together to function as one, held together by variety of attractive forces (same as tertiary)
Q10
secondary, tertiary, and quaternary structures
Q11
tertiary structures
Q12
hydrogen bonds
Q13
London dispersion forces, hydrogen bonds, dipole-dipole forces, ion-dipole interactions, salt bridges, and disulfide bonds
Q14
secondary, tertiary, and quaternary
Q15
No, a quaternary structure must have multiple subunits.
Concept Review Exercises
-
What is the predominant attractive force that stabilizes the formation of secondary structure in proteins?
-
Distinguish between the tertiary and quaternary levels of protein structure.
-
Briefly describe four ways in which a protein could be denatured.
Answers
-
hydrogen bonding
-
Tertiary structure refers to the unique three-dimensional shape of a single polypeptide chain, while quaternary structure describes the interaction between multiple polypeptide chains for proteins that have more than one polypeptide chain.
-
(1) heat a protein above 50°C or expose it to UV radiation; (2) add organic solvents, such as ethyl alcohol, to a protein solution; (3) add salts of heavy metal ions, such as mercury, silver, or lead; and (4) add alkaloid reagents such as tannic acid
-
Classify each protein as fibrous or globular.
- albumin
- myosin
- fibroin
-
Classify each protein as fibrous or globular.
- hemoglobin
- keratin
- myoglobin
-
A protein has a tertiary structure formed by interactions between the side chains of the following pairs of amino acids. For each pair, identify the strongest type of interaction between these amino acids.
- aspartic acid and lysine
- phenylalanine and alanine
- serine and lysine
- two cysteines
-
A protein has a tertiary structure formed by interactions between the side chains of the following pairs of amino acids. For each pair, identify the strongest type of interaction between these amino acids.
- valine and isoleucine
- asparagine and serine
- glutamic acid and arginine
- tryptophan and methionine
5. What level(s) of protein structure is(are) ordinarily disrupted in denaturation? What level(s) is(are) not?
6. Which class of proteins is more easily denatured—fibrous or globular?