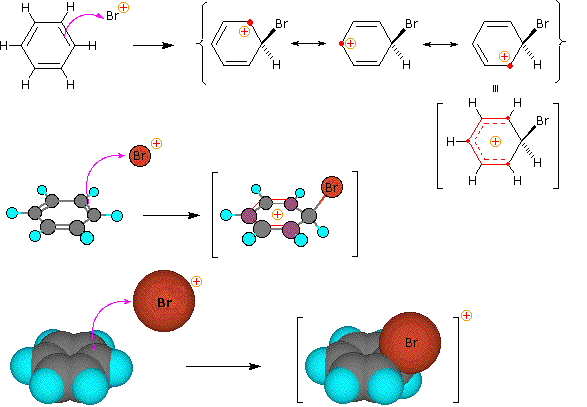8.2: Electrophilic Aromatic Substitution Reactions - Bromination
- Page ID
- 469415
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Halogenation is an example of electrophillic aromatic substitution. In electrophilic aromatic substitutions, a benzene is attacked by an electrophile which results in substition of hydrogens. However, halogens are not electrophillic enough to break the aromaticity of benzenes, which require a catalyst to activate.
A Mechanism for Electrophilic Substitution Reactions of Benzene
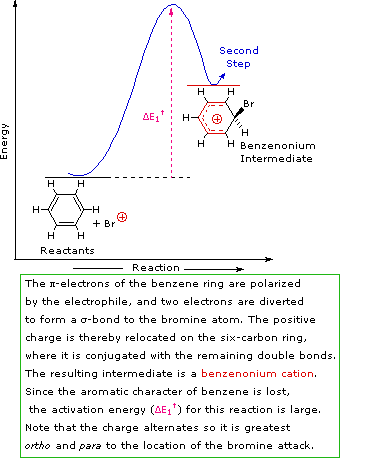
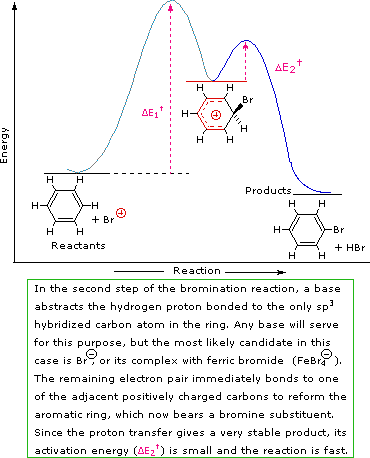
A two-step mechanism has been proposed for these electrophilic substitution reactions. In the first, slow or rate-determining, step the electrophile forms a sigma-bond to the benzene ring, generating a positively charged arenium intermediate. In the second, fast step, a proton is removed from this intermediate, yielding a substituted benzene ring. The following four-part illustration shows this mechanism for the bromination reaction. Also, an animated diagram may be viewed.
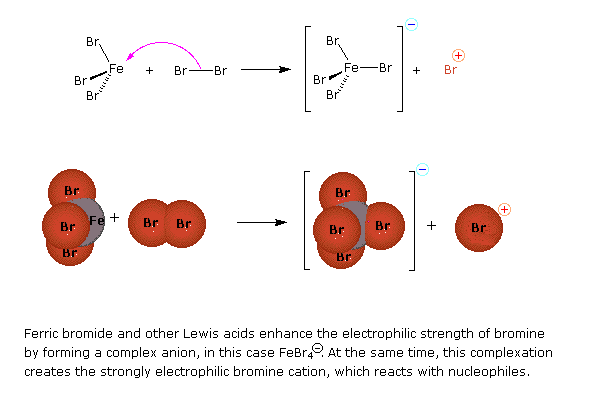
Preliminary step: Formation of the strongly electrophilic bromine cation
Step 1: The electrophile forms a sigma-bond to the benzene ring, generating a positively charged benzenonium intermediate
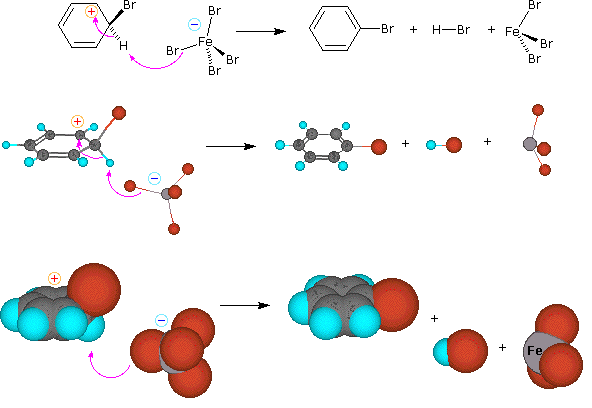
Step 2: A proton is removed from this intermediate, yielding a substituted benzene ring
This mechanism for electrophilic aromatic substitution should be considered in context with other mechanisms involving carbocation intermediates. These include SN1 and E1 reactions of alkyl halides, and Brønsted acid addition reactions of alkenes.
To summarize, when carbocation intermediates are formed one can expect them to react further by one or more of the following modes:
1. The cation may bond to a nucleophile to give a substitution or addition product.
2. The cation may transfer a proton to a base, giving a double bond product.
3. The cation may rearrange to a more stable carbocation, and then react by mode #1 or #2.
SN1 and E1 reactions are respective examples of the first two modes of reaction. The second step of alkene addition reactions proceeds by the first mode, and any of these three reactions may exhibit molecular rearrangement if an initial unstable carbocation is formed. The carbocation intermediate in electrophilic aromatic substitution (the arenium ion) is stabilized by charge delocalization (resonance) so it is not subject to rearrangement. In principle it could react by either mode 1 or 2, but the energetic advantage of reforming an aromatic ring leads to exclusive reaction by mode 2 (ie. proton loss).